The BF, which varies in shape and size according to poultry species, is opened by a small canal into the proctodeum region of cloaca
3,15,16. Mahanta et al.
3 reported that the BF was caudal 2.15±0.39 mm from the left kidney and 1.53±0.22 mm from the right kidney. In this study, BF of embryos according to certain embryonic periods were oval shaped. This finding was consistent with other studies
2,4,15,17.
Mahanta et al.3 reported that the average weight of the BF was 0.053±0.02 g. Also, average longitudinal diameter, transverse diameter and organ thickness of Kadaknath chick were 5.78±0.28 mm, 3.59±0.18 mm and 3.09±0.29 mm, respectively. In this study, the average BF weight was 0.006±0001 g on the 10th day, while this value was 0.037±0.007 g on the 21st day. Average cranio-caudal diameter was different according to the determined incubation days, statistically. While this value was 2.39±0.10 mm on the 10th day, it was 7.55±0.66 mm on the 21st day. The average latero-lateral diameter was ranged from 1.94±0.15 mm to 4.71±0.96 mm, while the average dorso-ventral diameter was in the range of 1.48±0.28 mm and 2.29±0.42 mm. These values indicate that the embryo and its organs develop and grow.
Optimal incubation conditions should be met in terms of reliability of the study. The relative humidity of the incubator. It is the water that evaporates from the pores in the egg shell that determines the relative humidity of the incubator. This also leads to daily egg weight loss. This rate should be 0.55%-0.70% during incubation18. In this study, the daily egg weight loss was between 0.53% and 0.67% (Table 1). These values were within the range of desired values, which indicates that our incubation conditions are optimal. In this study, average embryo weights were 3.07 g, 8.33 g, 24.27 g, and 41.19 g in the incubation on days 10th, 13th, 16th, and 21st, respectively. According to Table 1, the data were similar with the data of Bellairs and Osmond19. Differences may be due to egg size and/or chick breed.
In the present study, the highest relative embryo weight and the lowest relative organ weight were statistically 21-old-day embryos (P<0.05). Consequently, these data might be considered as an indication that the embryo developed.
As a result of the measurements, increased the BF weight and embryo weight were determined in the hatching time of chicks. The increase in BF weight and embryo weight depends on the development and growth of the embryo. It was determined that egg weights decreased as the hatching time of the chicks approached. Therefore, it was thought to be that the nutrients contained in the egg were burned with oxygen and converted into carbon dioxide and water and thrown out of the egg.
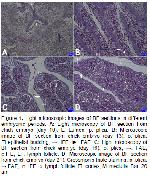
BF, a lymphoepithelial organ, is not only the primary but also a secondary lymphoid organ in terms of having a T-lymphocyte area in poultry. B-lymphocytes, which are responsible for the formation of a humoral immune response to diseases, develop and differentiate in this organ4,20,21. The epithelial bursa draft begins to develop in 4th-5th days. B-lymphocyte precursors migrate to this organ draft on the 7th day of incubation7,22,23. On the 9th day of embryogenesis, evaginations into the lumen of the mucosal layer are named as plica. In 10-day-old embryos, the development of lymph follicles is reported to be initiated by the accumulation of large basophilic cells under the epithelial layer24. In this study, a central lumen and plica development were observed on the 10th day of incubation in the bursal sections.
Dense basophilic cell accumulation forms epithelial buddings in the mucosa layer24. The wall structure of the BF consists of tunica mucosa, tunica muscularis and tunica serosa4. There are structurally different epithelium in the BF as FAE and IFE. Whereas FAE is pseudostratified columnar epithelium that covers the luminal surface of follicles, IFE is a single layer prismatic epithelium that covers inter-follicular regions25. In present study, 13-day-old embryos were found to have epithelial buddings, two different epithelial structures and three layers of wall in BF.
The development of the lymph follicle is reported to be completed in the organ by the 17th day of incubation. Each lymph follicle has a narrow cortex and a large medulla. The corticomedullar border, the continuation of IFE, separates the cortex and medulla10. In our study, the development of lymph follicle was clearly visible on the 16th day of incubation. On the 21st day, we were observed that histologic development of BF was completed. Advanced follicular development, marked separation cortex-medulla-corticomedullar zone, evident FAE and IFE development, and trabeculae were seen in the organ. According to the results of this study, embryonic development of BF was found to be consistent with the findings of some researchers4,22,26.
As conclusion, obtained results suggest that BF is an important lymphoid organ in terms of maintaining a healthy life of the poultry. Organogenesis and morphometric measurements of BF evaluated with this research by using chick embryos during incubation. It is thought that the data and information obtained from the present study may support the studies planned on this organ.