Cisplatin is a widely used antineoplastic agent with its proven efficacy and long duration of action in many types of cancer. However, it is known that there are restrictions in its use due to damage in organs such as testicles, liver and kidneys
21,22.
Oxidative stress is a very common mechanism in cisplatin toxicity, with the formation of free oxygen radicals depending on the dose and exposure time. These free radicals can initiate chain reactions in microseconds and programmed apoptotic cell deaths can be observed. The places where oxidative stress is most effective are the plasma membrane, mitochondria, nucleus, golgi complex and lysosomes. Free oxygen radicals cause a decrease in spermatogenesis and damage most organs and tissues along with the male reproductive organs. Spermatozoa membranes are particularly sensitive to oxidative stress damage due to their high level of poly-unsaturated faty acids. In addition, most DNA damage in spermatozoa is a result of oxidative stress. Also it has been reported that sperm quality decrease, sertoli, Leydig and germ cells, sperm count and motility decreases with cisplatin administration 23,24.
According to the sperm analysis results (Table 1), there was a significant decrease in sperm motility and density (P>0.05) and a significant increase in the number of abnormal head spermatozoa and abnormal total spermatozoa in the cis group (P<0.05). It is seen that these results support the literature information. Our opinion that these spermatological changes are caused by cell damage caused by the increase in ROS caused by cisplatin.
Chemotherapy and radiotherapy are the treatments that limit the antioxidant capacities of the cells and antioxidants and prooxidants are used considering the cell type, exposure time and environmental conditions. In studies conducted in rats, it was stated that MDA level increased due to oxidative damage with cisplatin application and GSH and CAT levels decreased. Various researchers have stated that natural and synthetic antioxidant substances such as Vitamin A, Vitamin C, melatonin, quercetin and royal jelly are effective against oxidative damage caused by cisplatin 22,23,25.
Yadav et al. 26 reported that lipid peroxidation increased, GSH, SOD and CAT levels decreased, in addition that degeneration in the seminiferous tubules, loss of germ cells and free radical-induced oxidative tissue necrosis occurred in the testicular tissue in histopathological examinations after 10 mg/kg cisplatin administration to rats. According to Simsek et al. 27 in their study investigating the effect of selenium against the damage caused by cisplatin in the testicles, they stated that selenium showed protective properties. Observed that the tubules had more regular areas, MDA levels were significantly lower than the cisplatin group.
Ilbey et al. 28, investigated the effect of melatonin against testicular damage caused by cisplatin. At the end of the study, they found that sperm count and motility increased, oxidative parameters improved, and Sertoli cells returned to normal as a result of melatonin application.
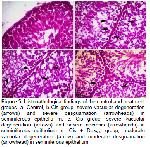
Figure 5 shows the degeneration, necrosis and desquamation that occurred in the seminiferous epithelium of the testes of rats given cisplatin in the histopathological examination of the testes. It has been observed that these findings are similar to the findings of the researchers mentioned above.
Ekinci Akdemir et al. 10, in their studies on the antiapoptotic and antioxidant effects of eugenol against cisplatin-induced testicular damage, it was observed that cisplatin increased testicular MDA levels and decreased GSH-Px, SOD, CAT values. In addition, dense spermatocytes in the walls of the seminiferous tubules decreased, severe degeneration and necrotic changes were observed in rats treated with cisplatin. In addition, edema in the intertubular interstitial areas, dilated veins in these areas and hyperemia were observed.
In our study, when the damage-relieving effects of the groups given boldine and dexpanthenol were taken into consideration, it was seen that there were serious improvements especially in the groups given 500 mg/kg dexpanthenol and 40 mg/kg boldine. Histopathological examinations of the tissues were evaluated, it is seen in Table 2 and Figure 5 that the tissue damage decreased in these two groups. A similar situation was observed in the spermatological examination results.
Büyütmek İçin Tıklayın |
Table 2: Testis seminiferous epithelial degeneration, seminiferous epithelial necrosis and desquamation scores in control and treatment groups |
Boldine is a natural substance with an alkaloid structure and has been reported to have a cell-protective, antitumor, anti-inflammatory and antipyretic effect 6. Dexpanthenol is an alcohol derivative of pantothenic acid (PA), also known as Provitamin B5, and is an important vitamin for the biosynthesis of coenzyme A 11. Pantothenic acid and its derivatives increase the level of glutathione (GSH), especially mitochondrial coenzyme A (Co A) and ATP, in cells and therefore play an important role in cellular defense, oxidative stress and inflammatory systems 14.
Fernández et al. 29 reported that Boldo (Peumus boldus molina) has an important antioxidant effect against oxidative damage caused by cisplatin and reduces the MDA increase caused by cisplatin. Previous studies, it was reported that the MDA level increased with hydrochloric acid administration and serum oxidative parameters changed with cisplatin administration approached normal with dexpanthenol administration. It has also been reported that the liver damage caused by cisplatin is reduced by the administration of dexpanthenol 14,22,30.
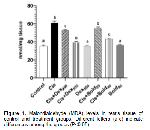
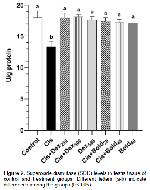
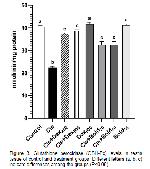
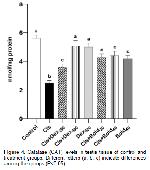
Oxidative stress parameters at the end of our study are shown in Figure 1, 2, 3, and 4 is investigated. It was observed in this study MDA level, one of the oxidative stress parameters, increased significantly in the cis group compared to the control group. However MDA levels decreased in other groups. The most significant reduction in MDA level was in the bold 40 and dex 500 groups. In our study, antioxidative enzyme levels (GSH-Px, SOD and CAT) were determined. The treatment boldine 40 mg/kg and dexpanthenol 500 mg/kg altered enzyme activities in cisplatin-treated rat testes tissue, but no significant change in enzyme activities was observed with at a dose boldine 20 mg/kg and dexpanthenol 250 mg/kg. In addition, similar results were obtained in sperm parameters. These results show that the effect of alkaloid on antioxidant enzyme activities becomes significant with increasing high doses. These results suggest that normalization of enzyme activities through ROS scavenging actions may have a significant contribution for the protective efficacy created by 40 mg/kg boldine and 500 mg dexpanthenol treatment. It was understood that these results were in line with the results of previous studies.
Changes in the amount of enzymes such as MDA, GSH-Px, SOD and CAT in testicular tissue are considered as indicators of oxidative damage. It has been reported that apoptotic and degenerative disorders may occur with an increase in abnormal sperm count, change in sperm density and motility, especially due to increased oxidative stress. 29.
As a result, we think that boldine and dexpanthenol may represent a promising new protective strategy against cisplatin induced testicular injury. And the use of boldine and dexpanthenol as an adjunct agent, may contribute to cisplatin used cancer chemotherapy. Future in-depth studies that may relate to these results will explore additional mechanisms and improve our understanding of testicular injury provided by boldine and dexpanthenol.