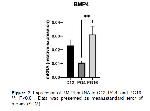
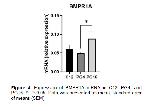
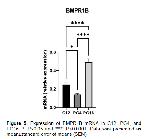
Our study apparently demonstrated that the mRNA expression of some BMPs genes were detected using qPCR in induced luteolysis (PG4 and PG16). The CL tissues obtained facilitated us to evaluate BMP2, BMP4, BMP6, BMPR1A, BMPR1B, and BMPR2 mRNA expressions in ovine CL.
Throughout the oestrous cycle, luteal regression in ruminants and other species is induced by the release of PGF2α, which reaches the CL from the uterus. Luteolysis is prevented when the embryo is present and emerges in cyclic animals 21,22. Endogenous or exogenous administration of PGF2α facilitates a signalling of events causing to irreversible death of CL. In the entire process, CL undergoes significant changes in terms of its steroidogenic capacity, vascularization, extracellular matrix regeneration and cell viability 23, 24.
In recent years, many functional genomic studies have elucidated the underlying mechanism of PGF2α over luteolysis 25, 26. Studies reveal that factors produced by uterine or exogenous PGF2α mediate a variety of processes from decreased steroid production to apoptotic cell death. Factors such as bone morphogenic proteins (BMP), tumor necrosis factor-alpha (TNFα), and activin A may have inhibitory effects on StAR expression 27-30.
It has been reported that some BMPs cause luteolysis by suppressing StAR expression and P4 generation in women and granulosa cells 13, 31. In our study, this may be consistent with the regulation of BMP genes that we showed BMP6 mRNA were upregulated in PG16 compared to C12. Also, we previously reported that StAR mRNA was shown to be sharply decreased in PG16, thus explaining BMPs inhibitory effects on StAR as identified previously in humans CL 13. BMP2 and BMP4 mRNA expression was demonstrated to be increased in induced luteolysis of cattle CL after 12 hours after PGF2α treatment, but BMP6 was after 2 hours 32. BMP2 abundance was reported to link/be linked with diminished P4 in cattle CL 33. However, we have not observed any changes in the expression of BMP2 and BMP4 mRNA levels in our study. BMPRs assessed in the current study were expressed in the ovine CL during luteolysis. We have not detected significant regulation for BMPR2 as in induced luteolysis of bovine CL 32. However, BMPR1A mRNA were not regulated in induced luteolysis against C12, whereas BMPR1B mRNA was upregulated in PG16. In line with our study, in rats, the greater levels of BMPR1B was reported in CL regression which implies its involvement in luteolysis 14.
In conclusion, considering mRNA expression of BMP2, BMP4, BMP6, BMPR1A, BMPR1B, and BMPR2, BMPs appeared to have functional role in corpus luteum. We may suggest that expression patterns of some BMP genes regulate luteolysis of ovine CL.