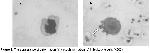
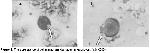
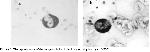
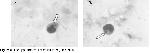
In this study, we investigated the morphological alterations in WBC of patients with lung cancer receiving RT. Histologically, small cell lung cancer was predominant (11 cases). Because, small cell lung cancer comprises 15–20% of all malignant lung tumors and in the majority of cases the tumor has disseminated or is locally advanced at the time of diagnosis and thus the rate of small cell cancer type is higher than the other lung cancer types. The exposure to γ–radiation caused some damages in WBC. These damages were observed in WBC, whereas such changes were not observed in erythrocyte and thrombocyte cells with light microscope. These damages might be due to direct exposure to radiation of cell or free radicals generated by γ–radiation. As a result, these free radicals cause breakage of chemical bonds, cross-linking and conformational changes in cellular components as lipid, protein and enzyme. Thus, these changes may affect the molecule's biological function and structure. Moreover, this structural damage can be explained by elevated temperature produced by radiation
17.
In our study, the MN frequency was also compared with WBC count. The MN frequency showed an increase during RT and WBC counts decreased significantly during RT. In all patients, the results showed that the highest frequency of MN was observed at the end of 5th week of RT and the lowest frequency of MN was observed before the initiation of RT. Moreover, the MN frequency in WBC was also elevated in cancer patients before RT when compared with the controls, and difference was statistically significant. Although these patients were not exposed to γ–radiation before RT, MN was observed in prepared slides. This result may be related to the patients' age, chemotherapeutic drugs taken before RT and cigarette smoking in particular. The effect of cigarette smoking on MN frequency was reported in many of the biomonitoring studies18–20. Besides, MN level was showed greater frequency in the elderly control group (non-smokers) with a mean age of 52.6 ± 2.9 years than the young controls with a mean age of 23.5 ± 1.3 years (non-smokers). MN formation was not observed in the young controls, but a low frequency of MN (0.05%) was determined in the elderly controls. The observation of MN in these healthy persons without RT and chemotherapy clearly indicates that MN formation is related to donors' age. These findings suggest that age can influence the formation of MN in WBC. The effect of aging on spontaneous MN frequency has been reported by various authors11,12,14,21–23. Higher frequencies of MN have previously been reported in females than in males24. However, the effect of sex difference was observed in our study.
If we consider the similar studies, our results are found to be in close agreement with previously published data. For example, Anna et al.25 investigated the level of cytogenetic damage in peripheral blood lymphocytes of patients undergoing chemotherapy. As a result, they showed that the highest level of cytogenetic damage was observed at the end of therapy. They determined the frequencies of increased MN during the first half of therapy and declined thereafter. Moreover, they observed the leukocyte count strongly decreased at the beginning of therapy with an upward trend at the end. Boreham et al.26 reported the relationship between radiation dose and radiation-induced the apoptosis and MN formation. They found that apoptosis and the MN frequency decreased in low dose rate of radiation, but apoptosis and the MN frequency in binuclear cells increased with increasing of applied radiation dose. Hubert et al.27 used MN test to determine the effects of radiation on 99 workers studied in Belgium Doel Nuclear Center. They reported an increase in the frequency of MN with increase in the annual exposure to radiation. In another study, MN formation and cell proliferation in human lymphocytes exposed to 50 Hz magnetic fields for 72 h was investigated. As a result, 50 Hz magnetic fields have no effect on MN formation, and a significant increase in cell proliferation was not observed28. Widel et al.29 investigated the frequency of MN in peripheral blood samples were taken before and after RT in patients with cervical cancer. As a result, they reported a significant increase in the MN frequency compared with the controls. Maes et al.3 reported effect of 2450 MHz microwave on the MN frequency in human blood lymphocytes in vitro. They determined an increase in the MN frequency with increasing of exposure duration. Rosin and Gilbert30 investigated the modulation of genotoxic effects of some chemical agents in humans by using MN test as a biomarker. Besides, Stich and Rosin31 reported the MN in exfoliated human cells as a tool for studies in cancer risk and intervention.
In conclusion, RT has a lethal effect on cancer cell. However, it may cause severe structural and genotoxic damages on healthy cells and tissues such as blood. These damages may also induce other disease such as leukemia and anemia32. Therefore, effects of RT applications on healthy cells and tissues must be minimized or alternative methods should be developed.