Animals: The local ethics committee approved our study protocol. Virgin female Wistar rats (200-220g) obtained from Firat University Biomedical Unit were used in this study. They were housed under controlled temperature (22 ± 1°C) and light conditions (lights on from 07.00-19.00 h). Food and water were freely available. Daily vaginal smear was performed and only animals on diestrus were included in the experiments.
Experimental Design: In chloral hydrate anesthesia (400 mg/kg, Botafarma Lab, Istanbul, Turkey), a carotid artery was cannulated and the animals were placed in a stereotaxic frame. A microdialysis guide cannula (CMA/Microdialysis, Stockholm, Sweden) and a microdialysis perfusion probe (CMA/Microdialysis, Stockholm, Sweden) were set into the right PVN with guidance of the rat stereotaxic atlas30. Artificial cerebrospinal fluid was run through a micropump (Harward App, Holliston, MA, USA) throughout the experimental period. The flow rate of artificial cerebrospinal fluid was set at 1.5ml/min. After a settling period (at least 1h), microdialysis samples were collected at 20 min intervals for a period of 80 min. In the first series of experiments, 50mg/kg CCK-8 (Sigma, St Louis, MO, USA), was administered to rats of the CCK group via the carotid artery cannula (n=8) after the collection of the first sample. In addition to CCK, melatonin (10mg/5ml; Sigma, St Louis, MO, USA) was infused into the left lateral ventricle to rats of the melatonin group (n=8). Animals of the vehicle (n=10) and CCK groups received intracerebroventricular (ICV) infusions of the vehicle. In the second series of experiments, the collection of the first microdialysis sample was followed by ICV infusion of 5µl artificial cerebrospinal fluid in the control, vehicle treated rats (n=8) and by infusion of ghrelin (1µg/5µl; Sigma, St Louis, MO, USA) in rats of the ghrelin group (n=8).
In all experimental groups, blood samples were collected via the carotid artery cannula concomitantly with the microdialysis sample at four time intervals. After collection of the last microdialysis and blood samples, rats were decapitated and brain tissues gently removed. Coronal brain slices were cut according to the rat stereotaxic atlas for verification of the location of the probes.
Chromatography: Catecholamine concentrations in microdialysis samples collected from the PVN were analyzed by a high performance liquid chromatography with electrochemical detector system (HPLC-ECD, Waters Corp., Milford, MA, USA). A 20ml aliquots of the samples were injected onto the HPLC column (ODS2, 4.6X250mm C18, Waters Corp., Milford, MA, USA) coupled to ECD. The concentrations of noradrenalin and its metabolite (3,4-dihydroxyphenylglycol, DHPG) were simultaneously detected. The method has been described previously31,32.
Oxytocin assay: Iodination of oxytocin was performed using chloramine-T. Material was chromatographed on a column of Sephadex G-25 fine (Fluka Chemie, Buchs, Switzerland). Radioimmunoassay (RIA) procedure per se was performed according to Robinson33 with 3 day incubation and separation of bound from free 125I oxytocin by addition of 900μl ethanol. Standard curves were obtained using 200μl dilutions of standard oxytocin (Peninsula-Bachem, Switzerland). 200μl samples of unextracted plasma were measured by RIA in duplicates with highly specific antibody kindly provided by Prof. Robinson (I.C., England). Intra-assay coefficient of variation was about 4.7%.
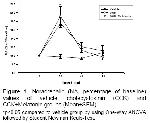
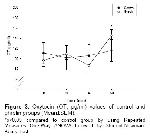
Statistics: Catecholamine data were evaluated and expressed as percentages of the baseline levels. Noradrenalin levels were normalized by nominating the control level as 100%, and its levels in 20-mins samples were expressed as percentage of these values. Data were statistically analyzed by two-way repeated measures ANOVA with post-hoc testing by using the Student-Newman-Keuls multiple range tests. p<0.05 was considered statistically significant.