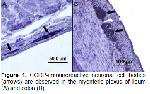
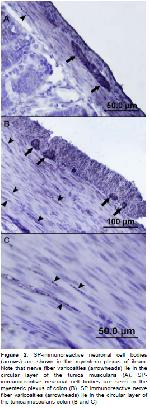
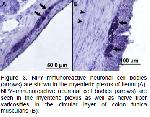
The enteric nervous system (ENS) is the intrinsic nervous system of the
gastrointestinal tract. It contains complete reflex circuits that detect the physiological
condition of the gastrointestinal tract, integrate information about the state of the
gastrointestinal tract, and provide outputs to control gut movement, fluid exchange
between the gut and its lumen, and local blood flow
1,2. There are several distinct
features of enteric neurons which contribute to their ability to regulate gastrointestinal
functions
3. The function of the ENS is to coordinate the complex interactions of the
enteric networks, which consist of sensory, inter, motor and secremotor neurons
4.
The ENS is usually formed from two major plexus: the myenteric plexus mainly
regulating muscle activity and the submucous plexus mainly regulating mucosal
functions. The myenteric plexus is an endogenous source for motor innervation to the
muscular layers and secremotor innervation to the mucosa. Similar to other nervous
systems, the functions of the myenteric plexus are mediated by various neurochemical
substances2,5,6.
The extrinsic and intrinsic components of the enteric nervous system appear to be
the primary mechanisms involved in motility regulation. It is well known that besides the
noradrenergic and cholinergic regulations, serotoninergic and peptidergic ones are
present, too7, some of them having a facilitatory action, others having inhibitory
effects upon classical neurotransmitters8-11.
Calcitonin gene-related peptide (CGRP) which was originally identified as a splicing
product of the alternative RNA processing of the calcitonin gene in the rat brain, affects
a variety of biological activities in the ENS, such as release of gastrointestinal hormone,
co-ordination of gastrointestinal motility, excitation of myenteric neurons and
vasodilatation12.
Substance P (SP) is a member of a family of peptides
called the tachykinins13 and a neurotransmitter that
plays an important role in regulating gastrointestinal
motility14. SP was isolated from the gut and can be
detected in a dense nerve fiber network within the
myenteric plexus. SP-containing nerve fibers are an
intrinsic contractor of the longitudinal muscle layer5,14,15.
NPY is widely distributed in the central and peripheral
nervous system and represents one of the most
abundant neuropeptides16. It has been functionally
related to regulation of blood pressure, circadian
rhythms, feeding behavior, anxiety, memory processing,
and cognition in the central nervous system, and to
vasoconstriction and gastrointestinal tract motility in the
peripheral nervous system17,18.
Although the immunoreactivity of the abovementioned
neurotansmitters has been studied in ENS of
several laboratory animals14,19-22, these
neurotansmitters have not been examined in small and
large intestine of mole-rats (Spalax leucodon). Thus, we
have investigated the location of CGRP, SP, NPY, VIP
and serotonin immunoreactivity in the extrinsic and
intrinsic innervation of the mole-rats (Spalax leucodon)
intestines.