Leishmaniasis is a zoonotic protozoa disease caused by Leishmania species and is endemic throughout the world
1,2. Due to its zoonotic nature, it is reported as a very important problem for both animal and human health, and it poses a significant risk for approximately 310 million people in 98 countries
2,3. Leishmania species are heteroxenous parasites and the sandflies act as its vector, while various mammal species act as reservoirs for the parasite
4,5. While its significance for the public health was unnoticed for many years, Leishmaniasis was included in the Tropical Diseases Research and Education Special Programme in 1976, which is a joint program of the World Bank and World Health Organization
5,6.
The disease has at least four main clinical forms, namely the visceral, cutaneous, mucocutaneous, and diffuse cutaneous forms 7-9. In Turkey, two forms of the disease caused by various Leishmania species can be encountered: the cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL) 8. Domestic dogs in urban and peri-urban areas and wild canids in the rural areas or nature are the main reservoirs for visceral leishmaniasis 10. Dogs are important for the spread of the disease as they can both clinically be infected and can act as reservoirs for other mammals, particularly humans 4,10,11. While the most common form of the disease is the cutaneous form, the visceral form is the most severe as it affects vital organs 5,6.
1.1. Etiology
The Leishmania species that cause leishmaniasis in the Old-World (Asia, Africa) is L.infantum, while in the New-World (America) it's L.chagasi 12. The complex life cycle of Leishmania, which is a digenetic protozoon parasite, consists of an intra-cellular phase which involves vertebrate hosts (rodents, dogs, humans) and an extra-cellular phase which involves invertebrate hosts 13.
1.2. Morphology
Life cycles of Leishmania species morphologically consist of two forms, namely the "amastigote" form encountered in the mammal hosts, and the "promastigote" form encountered in vectors 12,14. Amastigote form is non-flagellated, is oval or circular in shape, an is 2-5 μm in size. In the slides painted with Wright and Giemsa, the nuclei get painted dark red and are seen to be relatively large in size. Promastigote form is located at the intestines of the vector arthropod outside the cells and has a shuttle shape 15-20 μm in length and 1.5-3.5 μm in width 15-17.
1.3. Vector
In the New-World countries, Lutzomyia act as the vector for the parasite, while in the Old-World countries, the vectors are the Phlebotomus 5,12. Sandflies belong in the Phlebotomine sub-family of the Psychodidae (Phlebotomidae) family of the Diptera order of the Insecta taxon 18. More than 40 types of Phlebotoms and 30 types of Lutzomyias take part in the transport of the parasite 12,14. Leishmania parasites are only transported through the blood-sucking of the females of the Phlebotomine sandflies from the hosts. Today, a total of 988 sandfly types are identified, most of which belong to the Lutzomyia and Phlebotomus species, and 98 of these were identified as the vectors for various Leishmania species 18. Most adult vectors are 2.5 - 3.5 mm in length. The adults are in a variety of colors ranging from silver to almost black and are covered with tight and steep scales that give the impression of fur. It is a characteristic property that the wings of the adults take the V shape while in resting state. Their flight is weak, and their movements are characteristically in a hopping motion. During the day, the sandflies live in hot and humid places that have the suitable micro-climate and are protected from wind and rain, like residences, stables, coops, cracks on the walls, tree hollows, rodent nests, and caves 6,18-20. Vectors feed at dusk. Male and female sandflies feed on the sugar in the plants as a source of energy. Only females suck blood from humans and animals in order to mature their eggs 21. The lifecycle of the sandflies has four stages, namely the egg, larvae, pupa, and adult phases 8. Sandflies are usually spread between 50o North and 40o South latitudes, in the southern portions of Europe, and in Asia, Africa, Australia, and Middle and Southern America 18.
1.4. Lifecycle
As the Leishmania species complete their life cycle in two vector bodies one of which are vertebrate and the other one is invertebrate, they are classified as diheteroxenous parasites 13. Only female sandflies take part in the spread of the disease. Female sandflies need blood to mature their eggs. These flies take macrophages that contain the amastigote form as they suck blood from an infected organism. The amastigotes are released in the stomach of the sandfly and stay inside the body of the fly for 4 to 25 days during which they develop into promastigote form and multiply by simple division. These promastigotes then move to the proventriculus and then to the esophagus. As the vector sucks blood from a new organism, it also transmit the promastigotes. Once inside the body of the host, the macrophages phagocyte the parasite. While the macrophages try to destroy the parasite with lysozyme enzymes, the parasite is resistant to these and survives, and loses its flagella and transforms into amastigote form. The parasite begins to multiply by simple division, the macrophage is damaged, and the amastigotes spread and infect new macrophages, establishing a series of events which becomes a cycle 4,6,13.
1.5. Symptoms
Once the disease is contracted, different forms of the disease can develop, like asymptomatic, oligosymptomatic, and symptomatic forms. Amongst the symptoms most commonly encountered in dogs, the dermatitis is the most prominent one. The irregularities in hair and skin may be limited to surrounding tissues of eyes, nose, or the ears, or may be spread to the whole body 7,12,22. Clinical findings depend on the stage of the disease, the immunity status of the dogs, and the treatment applied to the patients, and 1/3 of the infected dogs display no symptoms 23,24.
It is possible that the dogs infested with the parasite will not display any clinical symptoms or will display one or more of the nine main clinical symptoms. These include skin lesions, weight loss or loss of appetite, local or general lymphadenopathy, ocular lesions, epistaxis, lameness, anemia, renal failure, and diarrhea. While the body temperature fluctuates, it generally stays at normal levels or proceeds slightly above the normal 15,24,25.
1.6. Diagnosis
Direct [Microscopic examination, Histopathology, Immunohistochemistry, Culture, Parasite isolation in laboratory animals, Xenodiagnosis, Polymerase Chain Reaction (PCR), Real-time PCR], indirect [Humoral immunity, Indirect immunofluorescent antibody test (IFAT), Counterimmunoelectrophoresis (CIE), Immunodiffusion assay (IDA), Direct agglutination test (DAT), Enzyme-linked immunosorbent assay (ELISA), ELISA-recombinant antigens, Immunochromatographic rapid tests, Western blotting (WB), Flow cytometry (FC)] and cellular immunity [Montenegro test, Lymphocyte proliferation assay (LPA), Interferon-g (IFN-g) cytopathic effect inhibition bioassay (IFNB)] methods can be used in the diagnosis of the disease 26. Similar to numerous other parasitic infestations, PCR can be used in diagnosis in leishmaniasis. The sensitivity of this technique for the diagnosis of leishmaniasis is significantly high (70-93%) 27. IFAT has a sensitivity and specificity approaching to 100%, and is thus accepted by the WHO by the reference serological test and is considered to be the golden standard method by some researchers 28-30.
1.7. Prognosis
The prognosis of the patient is closely related to the stage of the disease. While cutaneous forms can heal spontaneously, they may leave scars in healing areas. That being said, if untreated the visceral form which affects the internal organs may lead the patient to death, and the death still may occur despite the treatment, and thus this variant has a bad prognosis 15,31.
1.8. Treatment
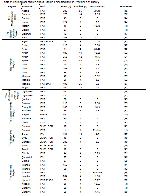
The first line of drugs globally preferred to treat leishmaniasis are the pentavalent antimonials. That being said, in cases where the disease develops resistance to these drugs, miltefosine, paromomycin, and liposomal amphotericin can be used in order to apply advanced combinations 32. Pentavalent antimonials are not only quite expensive but may also cause side effects. An antimonial treatment that exceeds 2 months is considered to be risky for patients with cardiac, renal, or hepatic deficiencies. Furthermore, long periods of antimonial treatment may cause the disease to develop resistance against the drugs 12. In recent years, sodium stibogluconate, meglumine antimoniate, pentamidine, amphotericin B, allopurinol, ambisome, and ketoconazole are frequently being used for the treatment of leishmaniasis in dogs 1. Oral miltefosine may be used in the treatment of patients that dont respond to antimonials 33. This medicine prevents the development of Leishmania species by blocking the Allopurinol RNA synthesis. Treatments with Allopurinol with a dose of 10-15 mg/kg/day for 2 to 4 months is reported to provide a cure, but relapses are possible afterward 12. The drugs used in the treatment of visceral leishmaniasis are listed in Table 1.
1.9. Prophylaxis
Early diagnosis and treatment, vector and reservoir control, and educational studies conducted in the endemic regions are the most important elements of protection 34. The disease caused by L. infantum is currently a threat to both dogs and humans. The asymptomatic dogs acting as reservoirs, and the symptomatic dogs which could experience relapses even after treatment, are the main concerns. For the sake of both human and animal health, early diagnosis and complete treatment of infected dogs is a necessity 35. In the fight against vectors, destruction of vector habitats (manure areas, garbage deposits, etc.), putting insecticide-treated (deltamethrin) collars on dogs, disinfection of animal shelters with insecticides, fight against rodents, and larvacide applications can lead to effective control 8,21.
2. Canine Leishmaniasis Studies in Turkey
While the seroprevalence of L. infantum varies between regions based on the ecological properties, it was reported to be similar in all Mediterranean countries and studies report it to be between 1.6% and 44.9% 36. The distribution of the disease positivity by region is given in Figure 1. Studies have been carried out to determine the seroprevalence of canine leishmaniasis in Turkey is given in Figure 2. The distribution of the disease in the country by province is given in Table 2 and distribution by region is given in Table 3.
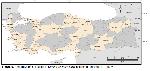
Büyütmek İçin Tıklayın |
Figure 2: Seroprevalence studies of Canine leishmaniasis in different province of Turkey |
In a study performed in the city of Manisa 37, the sera of 490 dogs were analyzed with the IFAT method, and the prevalence of the disease was determined as 5.3%. In another study, using the IFAT method Gönül et al. 38 reported that a 5-year-old Kangal dog was diagnosed with leishmaniasis. Another study that took place in Sakarya 39 reported the seroprevalence of the disease as 1.45%. Voyvoda et al. 40 report the seroprevalence of the disease in Aydın and İzmir as 10% and 2.5%, respectively.
Some studies report no seropositivity for a given region, like the study of Handemir et al. 41 which was performed in Istanbul. Kamburgil et al. 42 also reported no seropositivity for Istanbul in their own study as well. Similarly, the study of Babür et al. 43 report zero seropositivity for the 80 dogs they investigated with the IFAT method in the city of Şanlıurfa. While the study conducted by Beyhan et al. 44 in Burdur reported that all of the samples were found be seronegative, the serum samples of 124 dogs were inspected in another study by the same researcher in Hatay and the seroprevalence of the disease was found as 0.8%. Ica et al. 4 investigated a total of 300 serum samples collected from the dogs in Kayseri, all of which were found to be seronegative. Inspection of 120 serum samples collected from dogs in Dicle and Hani districts of Diyarbakır by Celik and Sekin 11 using IFAT method have revealed no seropositivity regarding the disease either. Kilic et al. 45, on the other hand, determined that only 2% of the 50 dogs inspected with IFAT method in the city of Sivas were seropositive. Tok et al. 46 also report as part of their study performed in Çanakkale, that all of the 27 serum samples collected from the dogs were found to be seronegative. Similarly, Aktaş et al. 47 report no seropositivity for the 72 dog serum samples they have evaluated in the province of Erzurum. The same is true with Icen et al. 48 where they reported zero seropositivity for the city of Diyarbakır. While the study performed by Bolukbas et al. 49 in Samsun reported seropositivity of 0.41%, all of the samples in other studies the same researcher reports for the cities of Amasya, Ordu, Tokat and Sinop provinces that all samples were seronegative. In a study conducted in Edirne by Düzbeyaz et al. 50, all of the 37 serum samples collected from dogs were found to be seronegative as well. All these studies were performed in various regions of the country and either report zero or very low seropositivity rates, and make up for the majority of the studies surveyed as part of this hereby study.
Certain studies, on the other hand, report seroprevalences as high as 27.5%, which is the case for the study of Doğan et al. 51 which reports that particular ratio for the city of Afyon. The same study claims the seroprevalence of the disease was 7.8%, and 9.09% for the cities of Eskişehir and Bilecik, respectively. Reporting another high seropositivity, Karakuş et al. 10 investigated serum samples of 206 dogs in Adana with IFAT method, and found the seroprevalence to be 27.18%. Similarly, Utuk et al. 3 conducted a study that covered the cities of Mersin, Kocaeli, and Sakarya, and detected the prevalence of the disease as 18.75%, 10.53%, and 5%, respectively. Of particular note is that the same researcher reports no seropositivity for all of the 37 dog serum samples inspected in in Elazığ.
It is also interesting to note that certain cities within the same region can have considerably different seroprevalences for the disease. Atasoy et al. 52 conducted a study that covers the cities of Aydın, Manisa, Muğla, and İzmir (all of which are within the same region), and report the prevalence of the disease as 14.1%, 3.8%, 12%, and 4.6%, respectively. The results also vary between the studies within the same region, as Bakirci et al. 53 conducted a study that covered Aydın, Manisa, and İzmir, and reported the prevalence of the disease as 21.95%, 9.52%, and 7.4%, respectively. In the same region, a total of 253 dogs were inspected with IFAT method in the study conducted by Töz et al. 54 in Aydın, and the seroprevalence of the disease was reported a 16.6%. The same researcher also used the same method in another study carried over in the province of Antalya where they evaluated the samples from a total of 109 dogs, and reported a seroprevalence of 9.1%. This number is considerably higher than the findings of another researcher 55 who performed a similar survey in the same province, and reported the seroprevalence as 2.58%. For various districts of the same province (Kepez, Kemer, Alanya, and Gazipaşa), Balcıoğlu et al. 56 performed their own survey studies and investigated the serum samples of a total of 176 dogs, and reported an average prevalence rate of 7.95%.
In a study conducted by Ertabaklar et al. 57 in Çorum, the serums of 131 dogs were inspected with IFAT method, and the prevalence of the disease was determined as 13.47%. In the neighboring city of Amasya, Gazyağcı et al. 1 investigated the serum sample of a single dog with IFAT method, and the dog was diagnosed with leishmaniasis.
İstanbul has the highest population amongst all the cities of Turkey, and while numerous researchers have reported no seropositivity for the city 41,42, Aysul et al. 58 reported a seropositivity of 1.96%.
Aydenizöz et al. 35 conducted a study in Kırıkkale and detected seropositivity of 2%. Dogan et al. 59 conducted a study in Eskişehir and examined the serums of 185 dogs with IFAT method, and detected a seroprevalence of 18.9% for the disease. Sari et al. 60 studied the serums of 165 dogs with IFAT method in their study conducted in Kars and detected the seroprevalence of the disease to be 7.27%.
As a result, this review has revealed the status of the Canine leishmaniasis in Turkey, which is a threat for both human and animal health. We believe this work will contribute greatly to the data regarding the spread of the disease across the country.