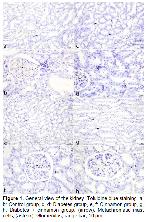
Mast cells are tissue-localized immune cells that play a key role in both innate and adaptive immunity as part of the physiological defense system
16. They have crucial roles in inflammation, autoimmunity, allergies, infectious diseases, and metabolic disorders
17. Chemokines, cytokines, growth factors, heparin, histamine, proteases, chymase, and tryptase, among other mast cell mediators, have a role in the pathophysiology of metabolic illnesses, including diabetes
18. In their study, Svensson et al.
19 showed that IgE levels increased in diabetes. At this point, this increase suggests that IgE-mediated mast cell activation may have an important effect on the pathogenesis of diabetes. Carlos et al.
20 reported that adaptive mast cell transfer before STZ administration confers resistance to diabetes and induces an increase in Treg cells in pancreatic lymph nodes. Also, they have stated that mast cells are associated with resistance to STZ-induced diabetes through an immunological tolerance mechanism mediated by Treg cells. In experimental studies, it was revealed that the number and activity of mast cells increased in the kidneys of rats with a diabetic model
21. In a study with diabetic rats, it was reported that the number of mast cells in the urinary bladder increased considerably. Furthermore, in the diabetic group in which Benfluorex - vitamin C treatment was investigated in the same study, fewer mast cells were found than in the diabetes group
22. Jones et al.
23 reported that the number of mast cell in the mesentery of diabetic rats increased statistically significantly compared to healthy rats. In the same study, it was revealed that antiallergic tranilast significantly reduced mast cell number in diabetic rats. It is well known that mast cells increase in diabetic rats, especially in the peripheral areas of the islets of Langerhans
24. Notedly, the previous study showed that a statistically significant increase in the number of mast cell in the ovarian and uterine tissues of experimental diabetic rats compared to the control group
25. In clinical and preclinical studies, it has been revealed that diabetes increases the density of mast cell and that agents that stabilize mast cells can improve diabetes experimentally
26. In addition, in our study, in which cinnamon extract also caused an increase in mast cells, it was observed that increased mast cells decreased in the diabetic group given cinnamon extract as a remarkable finding. This finding indicates that it might show an effective defense against possible damage to tissues and cells by diabetes through primary and secondary mediators in the granules of mast cells.
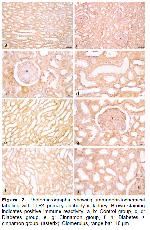
It is known that TLR4 has critical importance in host defense against bacterial infections 27. TLR4 has also been found to protect against inflammatory tissue damage by promoting tissue repair and remodeling in tissue injury 28. Research has shown that metabolic disorders such as hyperglycemia will damage kidney cells and encourage cells to secrete and release inflammatory factors 29. Gulden et al. 30 reported that the development of diabetes was accelerated, and Treg cell activity decreased in parallel in TLR4-deficient mice. In terms of inflammatory factor expression, it is known that TLR4 endogenous and exogenous ligands stimulate renal tubular epithelial cells in the case of hyperglycemia 31. Therefore, it can be thought that the expression of TLR4 in hyperglycemia may induce the production of a rapid and strong inflammatory response by renal tubular epithelial cells 32. TLR4 activation has been shown in one study to partially prevent insulin-related insulin resistance caused by a high-fat diet 33. In addition, one study has shown that insulin treatment can reduce blood sugar and TLR4 protein expression 34. It has been shown that TLR4 expression was increased in experimentally diabetic rat kidneys. Moreover, this research indicated a significant difference in TLR4 expression in the glomerular basement membrane, proximal convoluted tubule, and renal interstitial area of the kidney in rats in the modeling group compared to the control group 35. In our study, in parallel with the above studies, an increase in TLR4 expression was observed in the kidney tissue in the diabetes groups and the cinnamon-treated groups. In addition, when compared to the diabetes group, TLR4 expression was lower in the diabetes + cinnamon group. Based on the current knowledge and the findings of this study, we suggest that cinnamon may contribute to strengthening the immune system and, in turn, indirectly treat diabetes.
When mast cells are activated, they can release both newly synthesized lipid mediators, cytokines, chemokines, and preformed mediators like histamine from their granules. They then might cause an inflammatory response or participate in defensive responses due to this 36. The Fc receptors of mast cells express different receptors, including TLRs, chemokines, cytokines, as well as pathogen-associated molecular patterns of mast cell activation and immune responses 37. TLR4 receptors on the surface of human and mouse mast cells can regulate the FcRI-mediated mast cell signaling pathway in a synergistic manner, promoting mast cell degranulation and Th2 cytokine release 38. It has been demonstrated that TLR is expressed in both the cell membrane and cytosol of mast cells and can recognize viruses, bacteria, and fungi 39. A study reported that the number of mast cells and expression of TLR4 increased in the intestinal mucosa compared to healthy individuals with Crohn's disease 40. Huang et al. 41 stated that there were few scattered mast cells with TLR4 expression in healthy gingival tissues, but the number of mast cells and TLR4 positive mast cells increased significantly in the gingival tissues of the group with mild and severe periodontitis. In another study examining the relationship between mast cells and TLR4, it was stated that Giardia lamblia trophocytes, a parasite species, stimulated mast cells, and an increase in TLR4 expression was formed due to this stimulation 42. In a study using rats with necrotic enterocolitis in the ileum, it has been determined that breast milk oligosaccharides suppressed the mast cell number and TLR4 expression, and also reduced the damage in the ileum tissue 43. In our study, there was a parallel increase in the number of mast cells and expression of TLR4 in diabetes group and cinnamon group. Taken as a whole, this synergistic increase led us to suggest that mast cells might both secrete TLR4 and induce TLR4 expression.
These findings showed that cinnamon increases mast cell number and expression of TLR4 in healthy rats. At the same time, it caused a synergistic decrease in increased mast cell and TLR4 expression in rats with diabetes. In conclusion, we think that cinnamon, by activating or stabilizing particular cells and cytokines, can help avoid cell and tissue damage that may arise due to metabolic problems.