Research and Publication Ethics: This study was performed in accordance with the approved ethical rules of Atatürk University (protocol no. 2021/1, decision number: 30) and for each cattle written informed consent was obtained from the owner.
Animals and Protocol Design: The material of the study consisted of 42 cattle of 6-8 months old, Simmental and Brown Swiss breeds, and both genders. According to clinical examination findings and blood test results, the cattle were divided into two groups: Dermatophytosis (infected, n=28), and healthy (control, n=14). Cattle with dermatophytosis were divided according to the distribution of their lesions as mild (n=14) and severe (n=14). Thus, a second grouping was made. Cattle with any disease symptoms other than dermatophytosis were not included in the study.
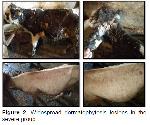
Mycological Examination: Cattle with circular, white, topical, or dense alopecic, and bran lesions were included in the study. While sampling from the skin lesions of the animals, the conditions of asepsis and antisepsis were complied with by using alcohol. Skin scraping samples were taken using a sterile scalpel tip and a sterile plastic brush using the brushing technique 12 and cultured on Sabouraud Dextrose Agar containing 0.5% chloramphenicol (HiMedia, Mumbai, India). Cultures were incubated at 25±1 ºC for up to three weeks and controlled periodically for fungal growth. The identification of isolated colonies was made according to the dermatophytes identification scheme. The macroscopic examination of colonies was recorded then cultures were stained with lactophenol cotton blue for microscopic identification and then visualized under the microscope. In the microscopic examination, the presence of septa, shape, size of hyphae, and conidial cells were evaluated under a light microscope for examination 13.
Blood Sampling: For haematological and biochemical investigations, blood samples from all the calves were taken from the vena jugularis externa and placed in tubes containing EDTA (Vacutainer, K2E 3.6 mg, BD, UK) and gel (Vacutainer, BD, UK). Blood samples were centrifuged at 3000 rpm for 10 minutes after waiting at room temperature in gel tubes. The sera were stored at -80°C until biochemical analysis.
Hematological Analyses: A hematology analyser was used to measure the cattle's white blood cell (WBC), lymphocyte (LYM), monocyte (MON), neutrophil (NEU), eosinophil (EOS), red blood cell (RBC), and hemoglobin (HGB) counts, and hematocrit (HCT) and platelet (PLT) levels (Abacus Junior Vet5, Hungary). The haematological assays were completed immediately.
Biochemical Analyses: Serum 25-(OH)D and PTH concentrations were determined by the electrochemiluminescence immunoassay (ECLIA) method using a chemistry analyzer (Cobas e801; Roche Diagnostics, Switzerland). A biochemistry auto analyser (Beckman Coulter, AU5800, USA) was used to analyse serum Ca and P levels using commercial kits.
Statistical Analyses: For statistical analysis, SPSS software (Version 25.0, SPSS Inc., Chicago, IL, USA) was utilized 14. To evaluate the data distribution between the groups, the Kolmogorov-Smirnov test was utilized (Infected and Control groups). Parameter comparisons were made using the Independent-Samples t-test between healthy cattle and cattle with dermatophytosis. One-way analysis of variance (ANOVA) and the post hoc Duncan test were used for statistical analysis of the subgroups; control, mild, and severe groups. The Pearson Correlation test was used to measure the correlation between the parameters. All results were presented as mean ± standard deviation (SD), and P<0.05 was used for all statistical comparisons.