Pregnancy toxemia occurs in over-conditioned sheep and goats carrying twins, triplets, or large fetuses during last 4-6 weeks of pregnancy
1. Goats are socioeconomically significant animals because they can adapt to a wide range of climatic conditions, can be grazed even in stony places that cannot be appraised in any other manner, and are especially preferred by family companies
2. Due to the herd-based nature of pregnancy toxemia, it results in significant economic losses due to the loss of both goats and offspring when preventive and treatment are not carried out appropriately. In the early stages of the disease, the majority of animals are asymptomatic or exhibit anorexia, dry and mucous feces, ataxia, and blindness. Some animals may exhibit convulsions and respiratory distress in the last stages, along with an inability to stand or lateral recumbency. If timely and proper treatment is not provided, the mortality rate is around 80%
3,4. The underlying mechanism of pregnancy toxemia is the inability of the pregnant ewe to meet the increased energy demands of the growing fetus during the final stages of pregnancy. To meet energy imbalance, body fat stores are breakdown to produce sufficient energy by gluconeogenesis. However excessive lipolytic activity results in an increase of keton bodies in the circulation. Fetuses cannot synthesis glucose to meet their energy requirements and are therefore dependent on glucose given by the maternal circulation
4,5. The underlying mechanism of pregnancy toxemia is the inability of the pregnant ewe to meet the increased energy demands of the growing fetus during the final stages of pregnancy. Body fat stores are broken down to produce sufficient energy. However excessive lipolysis results in an increase of ketone bodies in the circulation
6-8.
Vitamin B12, also known as cyanocobalamin, is a water-soluble vitamin and is an important part of enzyme systems involved in multiple metabolic pathways and energy production, mainly through rumen fermentation 9,10. This vitamin functions as a coenzyme and is involved in energy metabolism and cell replication 11. In particular, methylmalonyl CoA mutase is a cobalamine-dependent mitochondrial enzyme and plays a regulatory role in gluconeogenesis and fatty acid oxidation in ruminants 12. If the cobalt concentration in the ruminal fluid is below 0.5 mg/mL, the ruminal synthesis of vitamin B12 by ruminal flora is inhibited and its passage into blood and other tissues is reduced 10,13. When ration does not contain cobalt, B12 production in the rumen decreases within a few days, however, the vitamin B12 stored in the liver is enough for a few days 14. Synthesized cobalamin is absorbed from intestine into the the portal circulation and transported to the liver with folates. While some of the folates are utilized by liver cells, others are methylated and then released into the bloodstream for use by peripheral tissues 15. Similar to vitamin B12, folate is also a water-soluble vitamin, and has vital functions such as protein synthesis and red blood cell production 16. Unlike other water-soluble vitamins, approximately 60% of B12 is stored in the liver 17. In the case of B12 deficiency, it has been reported that folate concentration is depleted in liver cells 18. Excretion of absorbed vitamin B12 occurs via the urinary, biliary and fecal routes, but most is excreted in the urine 10,11,19. Serum B12 level can vary with factors such as feed, heredity, body reserve and herd management. In normal cobalt intake, serum B12 level is between 1-3 ng/mL. The most important factors for preventing vitamin B12 and folic acid deficiency are adequate and balanced feed intake as well as healthy rumen, liver, and kidney functions 20.
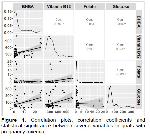
The studies on serum vitamin B12 and folate status in goat are limited. In this study, it was aimed to determine the beta hydroxy butyric acid (BHBA), B12, folate and glucose levels of Aleppo goats with pregnancy toxemia and to reveal their relationship with the disease and thus to contribute to prophylaxis and treatment.