In human studies and animal models, consumption of a high fructose diet seemed to be related to obesity, higher triglyceride levels, and insulin resistance
15. It has been shown that Wistar rats fed with 60% fructose diet for 6-8 weeks is sufficient to cause MS
16-18. In the light of these findings and studies, the model created with high fructose (60%) diet was deemed appropriate for the research.
At the end of 8 weeks, an increase in the body weights of the rats was observed in all four groups. However, there was no significant difference among the four groups regarding body weight. In one of the two different metabolic syndrome models, no statistical difference were detected between the groups in the weights of rats fed with 60% fructose after 12 weeks, while in another study, rats fed with 35% fructose were found to be heavier than the control group at the end of 16 weeks2,18. Our study and other studies show that the duration of fructose feeding, rather than the fructose content, causes a significant increase in body weight.
In a metabolic syndrome model performed in Wistar albino rats containing a 60% fructose diet, after eight weeks, an ARB application of telmisartan showed a significant decrease in AST, ALT, T-K, LDL-C, VLDL-C levels and a significant increase in HDL-C levels 15. In our study, obtaining similar results at the end of 8 weeks supports that ACEi and ARBs show the same effects on liver enzymes and serum lipids, albeit with a different mechanism. However, the fact that HDL-C levels did not change with enalapril administration in our study may be attributed to the small sample size in the study.
In another metabolic syndrome model created with a high-fat diet in 20 weeks in rats, losartan administration, an ARB, showed an increase in T-C levels, which was expected to decrease, and no significant changes were observed in creatinine clearance19. The fact that the metabolic syndrome model was made with a high-fat diet and losartan, an ARB, in the mentioned study may explain the differences with our study. In our study, it is a surprising result that serum creatinine values were found at such high levels in the fructose group. Despite the elevated creatinine levels, BUN did not increase in our study. This might be hypothesized that the creatinine elevation might be related to the decrease in tubular secretion rather than renal failure. It is also an interesting result that creatinine values were lower in the fructose + enalapril group than the fructose group in our study. The fact that there was no difference in creatinine levels in the group given enalapril alone compared to the control group suggests that the creatinine change associated with enalapril in rats may not be related to changes in intraglomerular pressure, so called RAS activation. The variable levels of creatinine, which increased in fructose, and decreased in the enalapril group, may be related to fructose effects on creatinine by mechanisms that we cannot explain.
In a study conducted in ZSF1 rats, which are genetically suitable for the metabolic syndrome model, enalapril applied at a dose of 60 mg/kg/day into the drinking water of rats for 32 weeks showed significant improvement in serum cholesterol and TG levels but no effect on serum glucose concentration20. In our study, even enalapril administration given at a dose of 10 mg/kg/day for only eight weeks caused an improvement in serum glucose concentrations. A high dose of enalapril in the previously mentioned study may suppress rats' energy consumption via ACE inhibition21. This suppression is thought to be related to the inhibition of adipocyte growth via AT-222.
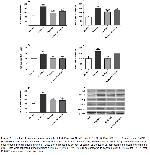
The studied IL-6, NF-κB, TGF-β, TNF-α, and SMAD-3 protein levels of rat kidneys were higher in the fructose given group than the control group. While enalapril administration caused a significant decrease in TGF-β, SMAD-3, IL-6 levels in the high fructose given group compared to the group given only fructose. However, enalapril administration caused a decrease in TNF-α and NF-κB levels; this decrease was not found statistically significant. In a metabolic syndrome model created with 60% fructose-fed rats for 12 weeks, significant increases in TNF-α, TGF-β, and NF-κB levels were observed in Western blot analyzes of liver tissues, and the levels of these inflammatory markers were found to be low in the group has given telmisartan, an ARB18. In our study, although significant increases were observed in the levels of TGF-β, IL-6, SMAD-3, NF-κB, TNF-α in kidney tissues in the fructose-administered group, NF-κB, and TNF-α levels were not significantly decreased with enalapril administration. This can be attributed to the relatively short duration of our experiment. In a study conducted with Sprague-Dawley rats, the administration of benazepril, an ACEi, significantly decreased TNF-α, TGF-β, NF-κB, and SMAD- 3/4 expressions in the heart tissue of rats compared to the control group23. The similar results obtained in our study can be explained by the fact that ACE inhibition stops the inflammatory process by inhibiting the intracellular pathways with AT-2 blockade24.
Metabolic syndrome is a chronic disease with various components and many complications caused by these components. MS causes severe morbidity and mortality all over the world. It harms the national economy by causing injuries and loss of workforce and the care and treatment expenditures created by complications. The positive steps to be taken in the treatment of metabolic syndrome will contribute to society and the state economy. Therefore, metabolic syndrome and related complications can be prevented with treatment modalities targeting inflammatory markers involved in pathogenesis. Our study showed that enalapril, an ACEi, shows promising results in treating complications such as diabetes, kidney failure, and obesity caused by metabolic syndrome. However, further experimental and clinical studies are needed.