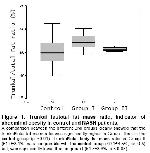
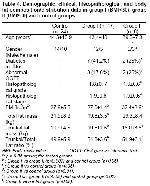
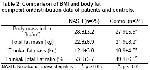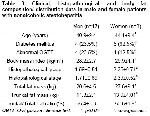The present study consisted of 25 consecutive
patients with NASH proven by histopathological
examination and had high alanine and aspartate
aminotansferase (ALT, AST) levels at Fırat University,
Gastroenterology Clinic.
The diagnosis of NASH was based on the following
criteria:
(1) Presence of steatosis (>10%), lobular
inflammation, and ballooning degeneration (with or
without fibrosis) on liver biopsy;
(2) Intake of less than 20 gr of ethanol per week, as
confirmed by the physician and family members who
were in close contact with the patient; and
(3) Appropriate exclusion of other liver diseases such
as alcoholic liver disease, viral hepatitis, autoimmune
hepatitis, drug-induced liver disease, primary biliary
cirrhosis, primary sclerosing cholangitis, biliary
obstruction, and metabolic liver diseases. No patient had a history of jejunoileal by-pass.
24 age-, sex-, and body mass index (BMI) matched
individuals who had normal abdominal ultrasound liver
scans, normal transaminase values, normal fasting
serum glucose levels, and normal glucose tolerance
tests served as the control group.
Informed consent was obtained from each patient
and the study protocol conforms to the ethical guidelines
of the 1975 Declaration of Helsinki as reflected in a priori
approval by the institution’s human research committee.
Local ethic comittee approved the study.
Histopathological grading and staging of the NASH
was made according to Brunt’s criteria 15 by a
specialist pathologist, who also made the
histopathological diagnosis of the NASH.
Grading: Grading was made according to
macrovesicular steatosis and necroinflammatory activity.
Macrovesicular steatosis:
Grade 0: No steatosis;
Grade 1: Steatosis up to 33%;
Grade 2: Steatosis between 33 and 66%;
Grade 3: Steatosis over 66%.
Necroinflammatory activity:
Grade 1: Mild;
Grade 2: Moderate;
Grade 3: Severe.
Staging: Staging was made according to fibrosis.
Stage 1: Zone 3 perisinusoidal / pericellular fibrosis;
focal or diffuse;
Stage 2: Focal or diffuse periportal fibrosis together
with Zone 3 perisinusoidal / pericellular fibrosis;
Stage 3: Focal and diffuse bridging necrosis together
with perisinusoidal / pericellular fibrosis and portal
fibrosis;
Stage 4: Cirrhosis.
Laboratory Analyses: Blood samples were collected
from patients and control group after an overnight
fasting. AST, ALT, total protein, albumin, alkaline
phosphatase, γ-glutamyl transpeptidase, HbsAg,
antiHCV, antinuclear anticor, smooth muscle antibody,
antimitochondrial antibody, serum cholesterol,
triglyceride, fasting glucose levels and complete blood
count were studied.
Patients with fasting serum glucose levels of more
than 126 mg/dl in at least two seperate samples were
identified as having diabetes mellitus, and a finding of 140-200 mg/dl two hours after the standard oral glucose
loading was considered abnormal glucose tolerance test.
As a measure of overweightness and/or obesity,
Body Mass Index (BMI) was calculated as the weight
(kg) divided by the square of height (m2) in all
participants and the patients with BMI of more than 30
were considered to show manifest obesity according to
the World Health Organization classification 16.
Patients divided into two groups according to the BMI:
Group I: BMI<30 (n=17) Group II: BMI≥30 (n=8).
DEXA measurements: Total and regional body fat
were taken at nuclear medicine department using Lunar
DPX-L scanner (Lunar Radiation, Madison, WI). The
data obtained from DEXA were further evaluated using
the Lunar 1.3 V program, which allows the operator to
determine specific body regions. The soft tissue
assessments were the total and trunkal fat mass (kg).
The trunk region consists of the area bordered by a
horizontal line below the chin, vertical borders lateral to
the ribs and oblique lines passing through the femoral
necks, includes chest and abdomen, excluding pelvis.
Abdominal obesity was estimated by the percentage of
trunkal fat, which determined by dividing the weight of
trunkal fat mass by the total amount of body fat 17.
Statistical analysis: Data were initially analyzed using
the MannWhitney U-test for independent samples and
the Kruskal-Wallis test for comparison among the
subgroups (Group I and II) and controls. The
relationships among the variables were analyzed using
Spearman correlation test. All analyses were performed
using the Statistical Package for the Social Sciences
(SPSS) for Windows, version 10. P values of less than
0.05 were considered statistically significant.