It was shown that ROS and other oxidants could be
also formed in the normal physiological process
2,3. It
is known that activated inflammatory cells cause ROS
production in BD a systemic, autoimmune disease
8.
Increased ROS, in turn, enhance LPO products, thus,
lead to tissue injury
2-7. H2O2 and other derivatives of
peroxides increase in some conditions, diffuse into
plasma. Here, antioxidant components of plasma
overwhelm them, and they are simultaneously consumed
15.
When TP is measured, it means that the sum of
many peroxides like protein peroxide, lipid peroxide and
H2O2 are measured 24. Although it is known that
H2O2 and lipid peroxides increase in BD 3,5-7,
oxidative stress has not been evaluated through TP in
BD. However, it has been reported that TP level
increases in passive smokers 25, preeclampsia cases
26 and cutaneous leishmaniasis 27. It was shown in
the present study that TP level also increased in BD.
Possible reasons for this increase in TP might be the
inevitable increase in lipid peroxides and ROS including
H2O2 in BD.
Many antioxidant molecules found in blood prevent or
inhibit the harmful effects of free radicals 15. Whenever
there is a decrease in antioxidants and/or an increase in
oxidants, oxidant/antioxidant balance is impaired in favor
of oxidants and this is known as oxidative stress 24,28.
It is known that oxidative stress is responsible for tissue
injury in many diseases and contributes to the
development of atherosclerosis 13,14. Antioxidant
activity indicates the antioxidant characteristics of only
one antioxidant, whereas total antioxidant capacity (TAC)
represents the total antioxidant characteristics of all
antioxidants found in the plasma. TAR and total
antioxidant status (TAS) are used synonymously with
TAC 28. It is doubtlessly more advantageous to
evaluate TAR, instead of individual antioxidant activities.
Many methods have been developed recently for this
aim. Total radical trapping antioxidant parameter (TRAP),
oxygen radical absorbance capacity (ORAC) and ferric
reducing antioxidant power (FRAP) are colorimetric
methods previously developed to assess TAC
16,17,28. It has been reported that TAR, a new
measurement method developed by Erel 16,17
correlated with data obtained by other measurement
methods and has had some extra advantages 16,17.
Blood has an important role in the oxidant/antioxidant
balance, as it carries and distributes antioxidants through
the body 28. Plasma has various antioxidant molecules.
Albumin, uric acid, bilirubin and ascorbic acid are the
major antioxidant components of plasma 16,17,28. TAR
represents practically all of them 16,17. Albumin
consists of about half of the TAC of the plasma 16,17.
Albumin has several biological functions, particular as a
ligand binder 29. Plasma thiol contents originate from
albumin. Thiol groups, on the surface of albumin, bind
oxidants 29. Low level of albumin can cause oxidative
stress via leading to increase oxidants like homocystein
29. In chronic inflammation albumin is reduced.
Bilirubin, a powerful endogenous antioxidant, is one of
the catabolites of heme oxygenases 30. However,
Harma et al. 26 have reported that bilirubin did not
correlated with TAR, in their clinical study. Uric acid is
another well-known low molecular weight water-soluble
plasma antioxidant 16,17. Uric acid has a strong
antioxidant activity and its concentration in the plasma is
about 10 fold than antioxidants like vitamin C and vitamin
E 28. In the present study, although there was a
significant increase in total protein levels in the BD
group, there was not any significant difference between
the BD group and the control group in terms of the levels
of such individual antioxidants as albumin, bilirubin and
uric acid. It has been reported that total protein, bilirubin
and uric acid levels correlated positively with TAC level
28. However, uric acid concentrations are influenced by
age, diet, heavy exercise, renal failure and some
metabolic diseases 28,31. Therefore, uric acid level
may not appropriately reflect the TAC. However, it has
been also reported that uric acid was not a strong
antioxidant and might not protect against free radicals
32. Noyan et al. 21 have reported that while vitamin C
levels reduced in BD oxidant MDA levels to be elevated,
on the other hand, there is no changes in the levels of uric acid.
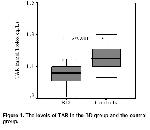
Orem et al. 6 have reported a decrease in TAR
level in BD. Similarly, TAR level was found low in the BD
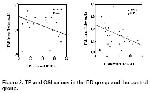
group in the present study. The increase in TP and OSI
levels and the negative correlation between these
increases and TAR level in the present study suggest
that a possible cause of the decrease in TAR level may
be increased oxidative stress. Since albumin, bilirubin
and uric acid levels were not significantly different
between the BD and control groups in the present study,
the decrease in TAR is not possible to attribute to only
these individual antioxidants. Therefore, the decrease in
TAR must have resulted from the decrease in other
antioxidants. It has been reported that activity of PON1
33 and level of SH 34, both of which are antioxidants,
decreased in BD. Besides, Ece et al. 35 have noted
that PON1 was positively correlated with TAR and
negatively correlated with TP and OSI in cases with
nephrotic syndrome. In the light of these data, a possible
reason for the decrease in TAR in BD may be the
decrease in other antioxidants, like PON1.
Plasma TAR level has been reported to be lower in
those with CVD, compared with those without CVD, in
smokers, compared with non-smokers, in diabetic cases, compared with non-diabetic cases, and in hyperlipidemic
cases, compared with those who have a normal lipid
profile 36. Additionally, children who are exposed to
passive cigarette smoking have been found to have a
decrease in TAR level, and an increase in TP and OSI
levels 25. It is known that oxidative stress is
responsible for pathogenesis of atherosclerosis 37,38.
As TAR is a fairly good representative of antioxidant
capacity, and TP and OSI are representatives of oxidant
capacity, decreased TAR and/or increased TP levels
indicate oxidative stress 16,17,28. In the present study,
there was a decrease in TAR level and an increase in TP
and OSI levels in the BD group. In the light of these data,
it is necessary to consider the possibility of development
of atherosclerosis while evaluating BD cases.
In consideration of the fact that increased ROS and
LPO products as a result of inflammation can be
responsible for the impairment in oxidant/antioxidant
balance, the increase in ROS and LPO products and the
decrease in antioxidant capacity are expected to be more
marked in the active BD group, in comparison to the
inactive BD group, due to marked inflammation.
However, no significant difference was found between
active and inactive BD groups with respect to TAR, TP,
OSI, and levels of individual antioxidants, such as
albumin, bilirubin and uric acid. This lack may be
explained by the activity criteria used to determine the
disease activity. It is known that currently there are no
agreed activity criteria for BD. It is also possible that the
oxidant/antioxidant balance is impaired at the onset of
the disease and continues. In this study presented here,
the fact that there is no relation between disease age
and oxidant and antioxidant markers in the BD group
may be supporting this theory.
In conclusion, TAR is an appropriate measurement
method demonstrating oxidant/antioxidant balance.
Oxidant/antioxidant balance seems to be impaired at all
stages of BD. In future, we think that this argument
should be confirmed by controlled, multi-centered,
prospective studies, which shall include large case series
and employ reviewed disease activity criteria.