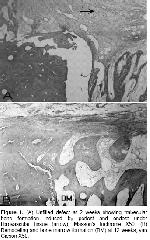
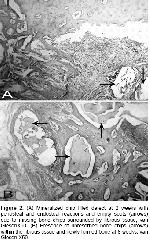
No foreign body reaction and postoperative complications were encountered in this study. Radiographic evaluation did not provide any means of useful information in the current study.
The healing of the unfilled defects as controls mainly through endosteal trabecular and less with periosteal bone formations because of superior immobilization of a circumscribed defect in the current study is in agreement with the reports of others 9,19. While depression which is the weakened area predisposing to cyst formation in the centre of the unfilled defects has been reported 9, there was no depression in our study at the end of 12 week period as reported by others 19.
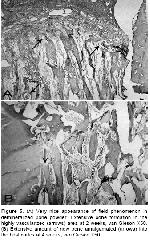
The remarkable histological difference of wide spread appearance of bone formation in demineralized xenogeneic bovine bone powder (Figure 5) compared with mineralized xenogeneic bovine bone powder, other forms and controls in our study, is compatible with the healing throughout the defect as a field phenomenon-induced bone formation in an orderly sequence of endochondral bone formation 16,17,20 utilizing demineralized allograft 18,20,21.
One must be cautious when talking about BMP because the results obtained through BMP may not be comparable to those of demineralized bone powder. The use of BMP extracted from demineralized bone and residuals itself loose and hampers the capability of induced bone formation 22,23. It is our opinion that, this is because demineralized bone powder has naturally everything in it and that BMP except one, lost every factor/factors that would be responsible to play a part to induce bone in a very complex environment. The problem of using BMP is the lack of an ideal delivery system 24. Taking into account these in demineralized graft, it also shows the importance of a suitable delivery system as the whole bone itself other than other delivery systems for BMP. At the present time, there is no ideal carrier to substitute bone powder and therefore, the demineralized bone powder is superior to deliver BMP with other preserved factor/factors.
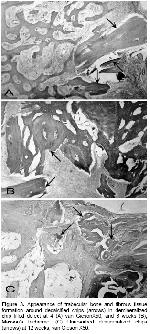
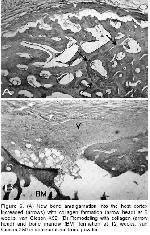
It was reported that unless supplemented with demineralized allogeneic bone matrix, autoclaved autoclaved autogeneic grafts were inferior 25,26. If this is the case as seen clearly in our study, that autoclaving per se show the potential of demineralized xenogeneic bovine bone powder for osteoinductive properties because in the autoclaved group, bony formation was suppressed as reported due to the inactivation of BMP 27. In our study, mineral containing implants were sterilized in autoclave which removed the any capacity of osteoinduction serving only as a scaffold and bone formation took place as early as 2 weeks like controls with almost no beneficial difference as to use of mineralized implant which is in agreement with observation of no new bone formation with the use of mineralized allogeneic powder and cortical chips for the augmentation of rat mandibular ridge even at 24 weeks 20. These results show the effects of active periosteal and endosteal bone formation in radial defects in our study. Bony formation was more suppressed in chip compared to powder form in the autoclaved grafts. At 2 weeks, the appearance of mineralized powder filled defects (Figure 4A) resembled of demineralized powder filled defects (Figure 5A) like field phenomenon most likely due to the smallness of the powder other than demineralization. This resemblance disappeared at 4 weeks and thereafter. This explains the importance of size in addition to demineralization in the early phase as early as 2 weeks. In the following weeks, the advantage of demineralization process in the powder form has become remarkable over mineralized powder. No resemblance was observed in either type of filled defects compared to the controls. This shows the importance of necessity of filling of the defects at least for the scaffolding effect.
Because in mineralized implants bone occurs through creeping substitution, it needs clarification that is there something to prevent the same type of bone formation in demineralized implants apart from the field phenomenon? In our study, excluding the healing of controls, occurrence of bone formation even at 2 weeks through creeping substitution with periosteal and endosteal bone formation in mineralized powder (Figure 4A) just like field phenomenon, may be considered as an obvious indication of additive healing that also occurred with demineralized bone powder (Figure 5A). Therefore, considering above and our results, it would be reasonable and reliable to say that bone formation occurs through mainly field phenomenon, secondarily to a lesser extent with suppressed periosteal and endosteal bone formation in demineralized xenogeneic bovine bone powder as in our study.
Longer exposure time to the acidic bath for the reduction of larger chips destroys the bioactive peptides decreasing the effectiveness of the chips to induce bone formation 16,21. Therefore it would be interesting to find out the critical size of the chips to obtain better results comparable to powder form in the future for demineralized implants. In addition to this, the formation of neovascularization is difficult with the use of large chips 16 which is in accordance with the findings in our study due to a barrier to vascularization 20.
Long-term resorption of demineralized allogeneic bone was reported 18. While mineralized xenogeneic bovine bone chips were resorbed by 8 weeks, demineralized bovine bone chips could still be identified at 12 weeks with no osteoclast observation around the chips in the current study. It was reported that induced osteogenesis had slow progression in demineralized allogeneic bone blocks as compared with allogeneic bone powder 16 which is in agreement with our results of demineralized bone chips undergoing less resorption with slowly proceeding osteogenesis in circumscribed radial defects. Presence of mineralized powder at the end of 12 weeks and absence of demineralized bone powder at the end of 4 week period along with formation of new bone much more in demineralized bone powder than mineralized bone powder at all time intervals may explain that demineralization promote new bone formation in powder form. In our study, resorption of demineralized bone powder at 4 weeks, in contrast to persistence of demineralized bone powder at 12 weeks as an onlay graft reported in a study 20 may reflect the effect of stress and compressional factors acting in the radius as being the long bone to decrease the resorption time in our study.
In our study, remodeling took place at 12 weeks in unfilled defect as well as mineralized chip and powder filled defects and demineralized powder filled defect. The advantage of demineralization process for the healing occurs as early as 2 weeks and it is lost or become equal to each other for those remodeled at 12 weeks. Therefore, regarding these and our results, if one prefers early bone formation in a particular defect should decide to use demineralized bovine bone powder due to superior rapid bony union compared to other forms.
Because induced bone is not species specific as was seen in this study and reported by others 17,28, wide variety of xenogeneic bone implant as far as animal resources are concerned makes the donor availability more versatile in contrast to allogeneic implants. It was concluded that demineralized xenogeneic bovine bone powder was superior to demineralized and mineralized bovine bone chips and mineralized bovine bone powder. It is obvious that demineralization overcome the disadvantages of the xenogeneic mineralized bovine bone powder and chips. Taking into account the ethical problems and difficulties as far as owner consent is concerned for the use of allogeneic bone for example (dog vs dog and cat vs cat), demineralized xenogeneic bovine bone powder may be a good choice to be used as an alternative to other types of grafts as being more versatile as animal resources are concerned.