This study revealed that the principal pancreatic islets of the Martes foina contained insulin, glukagon and somatostatin immunoreactive-cells. Additionally, somewhat different distributional patterns of these three types of immunoreactive cells were observed in the Martes foina.
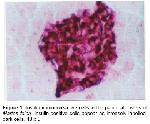
Insulin is synthesized in the B cells of the pancreatic islets and regulates the serum glucose levels 20. In the mammals, the regional distribution and relative frequency of insulin immunoreactive cells in the pancreas were reported in the hamster 10, C57BL/6 mouse 11, gerbil 13, wood Mouse 14, voles 21, opossum 22, and various laboratory animals 15. From these reports, it is well recognized that insulin immunoreactive cells are situated in the central regions of mammalian pancreatic islets and that other cells, such as glucagon and somatostatin immunoreactive cells, surrounded them. However, somewhat contradicting the finding of other researchers, Redy et al. 23 reported that these immunoreactive cells are observed in the majority of islets where they occur peripherally as groups of cells and within the pancreatic islets of several marsupial species. In the present study, most of the insulin immunoreactive cells were restricted to central regions of islets in the Martes foina, which is similar to previous reports on rodents 9,10,14,15,21.
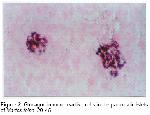
Glucagon is synthesized in the A cells of the pancreas and regulates blood glucose levels 20. Morphologically similar cells are also present in the digestive tract of the dog 15. In the present study, glucagon immunoreactive cells were mainly restricted to the mantle zones but a few of these cells were also demonstrated in the central zones in the pancreas of the marten. This results were found to be similar to that of mammalian pancreatic islets 9,10,15,21,22. Although it is seldom in rodents, cell clusters consisted of glucagon immunoreactive cells located in the connective tissue regions of pancreatic duct portions that are generally detected in higher mammals 24.
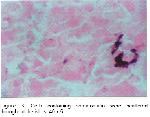
Somatostatin which consisted of 14 amino acid, was isolated from hypothalamus of sheep for the first time and it could be divided into straight form and cyclic form 25. This substance inhibits the secretion of the gastrin, cholecystokinin, secretin, glucagon, insulin, motilin and gastric acid 26 and absorption of amino acid, glucose and fatty acids in the gastrointestinal tract 27. In the pancreas of Martes foina, somatostatin immunoreactive cells were detected in the mantle zones and peripheral regions with moderate and rare frequencies, respectively. However, somatostatin immunoreactive cells were not demonstrated in the exocrine portions. This results are paralelled that somatostatin immunoreactive cells have been found in the outermost regions of mammalian pancreatic islets 9,10,14,15,21,22. However, in the present study, most of these immunoreactive cells were found in the mantle zones and mixed with glucagon immunoreactive cells. These presented topographically different distributional patterns in mammalian species 9,10,14,15,21,22.
In conclusion, the regional distribution of endocrine cells in the pancreatic islets of Martes foina was found to be similar to that of other mammals, especially rodents, exept for the topographically different distribution of somatostatin compared to that of other rodents.