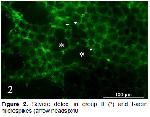
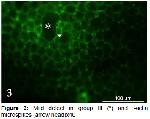
Animals and experimental design: A total of 21
healthy New Zealand Rabbits weighing 2.5-3 kg were
used in the study. Rabbits were kept in special cages
and maintained during the study period in Experimental
Research Center of Fırat University. Animals were
randomly divided into 3 groups 7 in each group. Group I
was control group (autologous serum), group II was
alkaline burn+saline solution (AB+SS) group, group III
was alkaline burn + autogenous serum (AB+AS) group.
Anesthesia technique: Rabbits in group II and III
were anaesthetised for the experimental procedure, the
rabbits were firstly injected with i.m. 5 mg/kg xylazine
hydrochloride (Rompun, Bayer, İstanbul, Turkey)
followed by 35 mg/kg ketamine hydrochloride (Ketalar,
Eczacıbaşı, İstanbul, Turkey). Analgesia was provided by
dropping 0.5% proparacaine hydrochloride (Alcaine
ophtalmic solution 0.5%, Alcon Laboratories, Istanbul,
Turkey) on the cornea before creating burn.
Creating alkaline burn: After right eyes of the
rabbits in groups II and III under general anesthesia were
opened with a Blepharostat , a filter paper with 6 mm in
diameter was immersed into 2 N NaOH and placed in the
center of the cornea and waited for 1 minute. The cornea
was then washed with saline solution for 2 minutes.
Preparation of autologous serum and
administration: After shaving of the outer surface of ear,
8-10 mL of blood was drawn from vena auricularis with a
vacutainer. The blood samples were centrifuged at 4000
rpm for 10 min to obtain autologous serum. Obtained
serum was stored at +4 ºC until use.
Three drops of non-diluted autologous serum were
dropped in the eyes of rabbits in group I.
Three drops of saline were dropped in the eyes of
rabbits with corneal burn in group II
Three drops of non-diluted autologous serum were
dropped in the eyes of rabbits with corneal burn in group
III. All administrations were made 4 times a day.
Applications were continued for 21 days in all three
groups
Histopathological examination: Rabbits were
decapitated at the end of 21th day. Removed corneas
were washed twice with phosphate-buffer saline (PBS).
Afterwards, they were fixed with 3.7% paraformaldehyde-
PBS solution at room temperature for 10 min.
Endothelium layer of the corneas were washed with PBS
again, and they were removed together with descement
membrane using microscope knife under Stereo
microcscope (Olympus SZX16). Removed layer was
washed again twice with PBS and then placed on the
microscope slide. It was held for 3 min at -20 ºC acetone
slides and washed with PBS several times, and were
incubated with 1% mixture of bovine serum albumin
(BSA)-PBS for 10 min before fluorescence staining. N-(7-
Nitrobenzofurazan-4-yl) phallacidine (Sigma - Cas
No:73413-78-2) was used for fluorescence staining.
Staining was carried out according to manufacturer
instructions. Methanolic phallicidine solution was
obtained by adding 1.5 mL methanol on 300 U
phallacidine. Each slide was incubated with 5 μL
methanolic phallacidine + 200 μL PBS solution at room
temperature. Slides were then examined under
fluorescence microscope (Olympus BX51) (x40). Seven
fields were randomly chosen from each slide and they
were examined. Defective areas were classified as
severe +++ (7 or more), moderate++ (between 4-6) and
mild+ (between 1-3 defect areas).
Horizontal and vertical lengths of the defective areas
in groups II and III were measured morphometrically.
Similarly, endothelial cells in 1 mm2 of each slide in all
three groups were counted8,13.
Statistical analysis: The data are expressed as
mean±SEM. P<0.05 value was considered to be
significant. Vertical and horizontal defective areas in
groups II and III were compared by sample t-test. The
differences in endothelial cells in all three groups were
analysed by one-way analysis of variance (ANOVA) and
post hoc. Tukey test SPSS statistical program (version
15.0) was used for the analysis of data