PPR has spread into many African and Middle East
countries
1,7,19,20 depending on the density of
animal movements, after it has first been defined in goats
in 1942 at Côte d'Ivoire
18. The disease was first
defined in Turkey in 1993 with pathological and
immunohistochemical findings in sheep
11. Later, it
was reported in sheep and goats in different regions of
Turkey with characteristic and introductory findings
10,14,16,17,21-25.
PPR infection in sheep and goats is observed in
three forms being hyperacute, acute and subclinical26.
Bronchointerstitial pneumonia usually occurs in natural
and experimental PPR infections6,8. It has been
reported that bronchopneumonia is macroscopically
characterized with consolidation and atelectasis and the
anterior and the cardiac lobes of bronchopneumonic
lungs are particularly dark red in colour and hard in
consistency8. In the present study, PPR viral antigens
were also observed in three cases which had catarrhalpurulent
pneumonia with mild consolidation. Although no
clinical examinations were performed in this study, the
three cases were considered as subclinical PPR infection
due to the presence of mild consolidation and mild
histopathological lesions in the lungs.
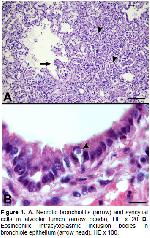
Microscopic lung lesions in PPR infections are
characterized with bronchitis, bronchiolitis or
bronchointerstitial pneumonia, viral inclusion bodies and
proliferation of epithelial cells in the respiratory tract7,8,10,11. The syncytial cells in the alveoli and the
bronchiole lumen and the intracytoplasmic inclusion
bodies in the trachea, bronchi and the bronchiole
epithelium are considered as pathognomonic for PPR2,8. In the present study, there were necrotic bronchitis,
broncholitis, interstitial pneumonia, syncytial cells and
inclusion bodies in some samples. However, in the three
cases in which PPR viral antigens were detected by the
IHC method, there were no necrotic changes, inclusion
bodies or syncytial cells in the respiratory tract
epithelium. Similarly, it has been reported that
eosinophyllic intracytoplasmic inclusion bodies and
syncytial cells are seen in some bronchopneumonias16. Yener et al. reported that the absence of the viral
inclusion bodies in the respiratory tract epithelium of
goats with PPR-related pneumonia may be due to the
status of the animals in the acute or recovery period of
the disease10. In an experimental study, it was stated
that syncytial cells may be identified through the last
stage of the infection27. On the other hand, the
inclusion bodies and syncytial cell formations observed in
lungs with PPR virus infections have also been reported
in parainfluenza (PI) and respiratory syncytial virusoriginated
infections4,6,28. Furthermore, it has been
stated that these lesions are suppressed when the secondary bacterial infections are involved6; therefore,
it has been reported that the PCR and IHC methods may
be used for the definitive diagnosis of PPR virus
pneumonia23,27. Similarly, it has been emphasized
that identification of PPR viral antigens is necessary for
the differential diagnosis24.
It has been previously reported that the PPR virus
may cause impairment in the mucociliary barrier or the
macrophage defence system as in PI type 3 virus
infections in cattle6. However, the results of molecular
studies have disclosed that Pasteurella spp. is
secondarily involved in the pathogenesis of the lung
lesions following invasion of the PPR virus to the
pneumocytes29. Similarly, PPR induced-pneumonia
may develop into a bacterial pneumonia that results in
death and the primary viral aetiology may be overlooked2,7-9,11. In the present case, the presence of oatformed
leukocytes in fibrinous pneumonia and diffuse
neutrophil leukocytes in catarrhal pneumonia were
recorded as the histological changes that show the
development of the secondary bacterial infections in
lungs, with the pulmonary defence system being affected
by the PPR virus.
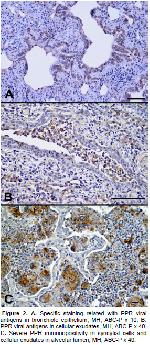
Because of its strong cross reaction in previous
immunohistochemical studies, polyclonal rabbit antirinderpest
serum was fixed with formaldehyde as the
primary antibody and it was successfully used for
identification of the PPR viral antigens in paraffin
embedded tissues8,10,11,16,17,24. In this study,
among 152 natural pneumonia cases, seven (4.61%)
had PPR viral antigens by the IHC method using
polyclonal rabbit anti-rinderpest serum. In recent regional
studies in Turkey, the rate of PPR-positivity has been
found to be 40% in goats with pneumonia10 and
11.42% in sheep with pneumonia24. On the other
hand, in another study, the prevalence of PPR infection
was determined between 0.87 and 82.6% and it was
higher in sheep (29.2%) than in goats (20%)14. It has
been stated that animal movements may be effective in
the differences in prevalence, as well as many factors
such as climate, infection time, virulence, the virus
amount, age and species of the animal, care and
nutritional conditions4,14.
In conclusion, the rate of PPR viral antigens in goat
with pneumonia in the Elazig region was found to be
4.61% by IHC. It has been concluded that PPR viruses
may be regarded as one of the aetiological agents in
goat pneumonia. Furthermore, when non-specific
histopathological lesions in the pneumonia with PPR
origin, as evidenced in the presented study, are
considered, the immunohistochemistry can be used as
an alternative method to advanced laboratory methods
for diagnosis of PPR infections in goats.