DEHP is well known for its testicular toxicity in laboratory animals
12,21,22. This toxicity caused a decrease in the relative testis weights with the body weight in experimental studies
23,24 also a decrease in GCLT and in the average ST diameter
25. In this study, DEHP administration significantly caused decrease in GCLT and in the average ST diameter with the weights of body and testes. These findings are supported by previous studies
23-25.
The lipid peroxidation (LPO) induced with toxic substances indicates the presence of oxidative stress in tissue with high concentrations of MDA, low concentrations of GSH9,26. The previous studies related to DEHP toxicity indicated that it was free radical trigger which caused increase in GSH-Px and CAT enzymes together with decrease in SOD and GSH levels2,27. In this study, biochemically the determination of increase in the concentration of MDA, decrease in GSH level, increase in the enzymes levels such as GSH-Px and CAT indicate that our findings were similar to the findings in the literature.
Many studies stated that DEHP toxication negatively affected testosterone production of Leydig cells22,28,29. Miura et al.30 indicated that the concentration of testosterone significantly decreased in association with Leydig cell dysfunction in DEHP treated animals. In addition, it was reported that DEHP toxicity caused to a decrease in the antioxidant enzyme activities, and an increase in the ROS and lipid peroxides22. Leydig cells are sensitive to the extracellular sources of ROS leading to ultrastructural changes in their structures31. In accordance with literature data given above, it is thought that the increase in the lipid peroxide levels and the inhibition of the testicular steroidogenesis caused Leydig cell dysfunction. Akingbemi et al.21 reported that Leydig cell hyperplasia induced as a result of DEHP exposure and this was associated with serum testosteron and LH levels. During experiment of our study, it was observed that there was a significant decrease in testosterone levels of DEHP treated group compared to the others. But, it was seen an increase in 28th day compared to previous experiment periods. It has been thought that these changes in testosterone levels which may be depend on Leydig cells dysfunction and hyperplasia.
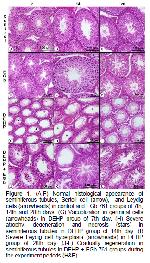
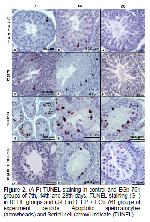
A repeated administration of DEHP was reported to cause testicular DNA damage5,12,23. The apoptotic cell indices of DEHP groups were found to be significantly higher than those of the control groups in our study.
Ablake et al.32 reported that DEHP firstly caused the spermatogenic deterioration and focal degeneration. Then, It was determined spermatogenic arrest found in Sertoli cells together with just a few spermatogoniums, but there was regeneration in the some tubules at the end of the experimental process. Additionally, while Park et al.12 suggested that DEHP toxicity caused edema in interstitial region and giant cells with the multinucleate with germ cell origin, the other researchers argued that DEHP induced hyperplasia in Leydig cells21,33,34. Similar to the literature findings in our experimental study; it was observed that the testicular damage and interstitial edema began in 7th day of the treated, this damage was still more deepened and multinucleate giant cell formation seen on 14th day. It was also observed that degenerative effects and Leydig cell hyperplasia to be more intense on 28th day. But there were a few tubule regeneration. Testicular damage observed in this work may be elucidated with the direct or indirect effect of DEHP, which later induces lipid peroxidation that is a chemical mechanism capable of corrupting the structure and function of testis.
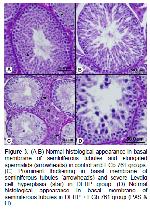
EGb 761 has eliminated the testicular damage with variety mechanisms such as stabilized the membranes and prevention LPO35. Toxicity studies showed that EGb 761 protected testis by suppressing ROS production and combating antioxidant enzyme deactivation against oxidative stress. In other words, it was determined that EGb 761 treatment neutralized the abnormalities in both MDA and the GSH levels, also transformed to normal the antioxidant enzyme activities such as SOD, GSH-Px and CAT8,36. In this study significant decreases in MDA level, GSH-Px and CAT activities and marked increase in rGSH level of EGb 761 treated rats was observed. These findings demonstrate that EGb 761 has a potent antioxidative effect.
It was reported that EGb 761 was a herbal agent which has a suppressive role against the sexual function dysfunction37 and increased the sexual performance by increasing their testosterone levels in the young rats38. In addition, it was stated that the testosterone level decreasing the result of Leydig cell damage in diabetic rats gradually increased with EGb 76139. This study showed that DEHP induced the decreased testosterone levels increased in EGb 761 treatment.
Kanter40 demonstrated that the decrease in ST diameter and germinal cell degeneration composed with ischemia / reperfusion injury approached to the control group with EGb 761. EGb 761 administration to DEHP treated rats significantly increased in ST diameter and in GCLT in parallel with the literatures in our study. This result may be associated with beneficial effects of EGb 761 being the spermatic cell proliferation in tubules.
The previous studies indicated that EGb 761 has antiapoptic properties13,40. Yeh et al.8 stated that EGb 761 pressured doxorubicin induced germ cell apoptosis and had antiapoptotic effect on testis. Also, it was determined that the ischemia / reperfusion and the testicular torsion increased the germ cell apoptosis and decreased these negative effects with EGb 7617,13,40. In this study, it was determined that DEHP induced testicular apoptosis gradually improved depending on antiapoptotic properties of EGb 761.