Plant Collection and Identification: Tridax procumbens leaves were obtained from open space adjacent to Department of Aquaculture and Fisheries Management, University of Ibadan, Nigeria. The plant was authenticated at the herbarium of the Forestry Research Institute of Nigeria (FRIN), Ibadan and a voucher specimen was placed under FHI110831.
Preparation and Extraction of Plant Materials: The plant was rinsed with distilled water and aired at ambient temperature for 2 hours. Five 5 g of T. procumbens fresh leaves was weighed on a digital ScoutPro sensitive scale in the Department of Aquaculture and Fisheries Management, University of Ibadan, covered with sterilized cotton wool and transferred into Soxhlet apparatus as described by 11,15. Briefly, about 170 mL of ethyl-acetate were poured in a spiral tube of the equipment. The extraction last for six hours until no further drop of extract in triplicates. The filtrate were concentrated on a rotary evaporator at 45°C for chemicals elimination, stripped into a sterile bottle and kept in a refrigerator until use.
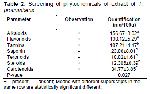
Phytochemical Analysis: The extracts of fresh leaves of T. procumbens were both qualitatively and quantitatively examined for the presence of secondary metabolites using various analytical tests and reagents. The metabolites examined were Alkaloids 16-17, Flavonoids 17, Tannins 18, Saponins (18), Terpenoids 19, Steroids 20 and Carotenoids 21.
Media Preparation: MacConkey, Nutrient, Plate Count Agars, nutrient broth and peptone water were prepared according to Manufacturers instruction (all agar were LAB-M products).
Microbial Analysis: The organs (liver, kidney, gill, intestine, flesh/muscle and spleen) were aseptically collected and weighed into sterile universal bottles while skin samples were collected using skin swab. The Samples were inserted into peptone water (0.1%) and allowed to release the available bacterial for a period of 2-3 hours. One mL was taken from each sample bottles and diluted in ten folds and subsequently serially diluted with dilution factor 10-4. Two ml were taken from each sample and dispensed into two petri dishes (1 mL to each). The first dish received plate count agar (PCA) for total viable count (TVC) (LAB M, LAB149) while the second petri dish received MacConkey agar (LAB M, LAB002) for total coliform count (TCC) using the pure plate count method. The media were prepared according to manufacturers instruction. Each dilution was overlaid with PCA and MacConkey respectively that has been cooled to 500C. At this temperature, agar is still in liquid form. The dishes were then gently swirled to mix the bacteria with the liquid agar 15. The mixtures were allowed to harden. When the mixture is hardened, the individual cells are fixed in place and incubated (Newlife Laboratory Incubator NL-9052-1) for 24 hours at 37 ⁰C to allow a distinguish colonies to form. The colonies formed were counted using Wincom Colony Counter (16W, 220V±10%, 50 Hz). The experiments were replicated three times. The total viable count and total coliform count were expressed in Log10CFU/g 6,21.
Antimicrobial Assay: Five pure bacteria isolates were obtained from Laboratory of Department of Microbiology, University of Ibadan, Nigeria. The isolates were: Listeria monocytogenes, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aereus and Aeromonashydrophilia.
Detection of Antagonistic Activity: Agar well diffusion methods were adapted. From the extracts, 0.1 g were dissolved in 2 mL of Dimethyl Sulfoxide (DMSO) (22) and reconstituted to have 50, 40, 30, 20, 10, 5, 2.5, 1.25, 0.625 and 0.3125 mg/mL. A cork borer of 6 mm diameter was used to create wells on the plates. Each well was filled with the extracts at the working concentration sterilized droppers and incubated at 37 ⁰C for 24 to 48 hours. The zones of inhibition were measured and minimum inhibitory concentrations were determined.
Feeding Trial:
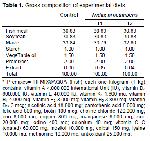
Feed Preparation: The best two concentrations that produced better zones of inhibition were incorporated in to feed formulated at 40% crude protein and fed to fish for 54 days. Organs were asceptically collected and examined for TCC and TVC. The feed ingredients (Table 1) were pelleted (2.0 mm) and packed in a polythene bags and store in a cool dry place until use with a well label on it.
Experimental Procedure: A total number of one hundred and eight (180) Heterobranchus bidorsalis juveniles (12.20±1.12 g) were used for the experiment. The fish were acclimatized for 2 weeks in plastic tanks before the experiment. Fish were weighed and distributed in to nine rectangular plastic tanks (60 x 38 x 27 cm) tanks in a completely randomized design. Each tank contained twenty fish and water was replaced on three days interval. The experiment had three treatments containing 0.0% (control), 0.05% (T1) and 0.04% (T2) of T. procumbens extracts in triplicates. The fish were fed at 3% body weight daily (1.5% was given in the morning by 8:00 am and 1.5% in the evening by 5:00 pm) throughout the experimentation period of 54 days. Water quality parameters were measured while the experiment lasted for fifty-four days. The protocol of this study was subjected to ethical consideration and received approval from University of Ibadan Animal Care Use and Research Committee (UI-ACUREC; U.I.ACUREC/IA/16/0005-UI-ACUREC/App/03/2017/008)
Statistical Analysis: The data obtained were subjected to descriptive statistics and one-way analysis of variance (ANOVA) at α0.05 using SPSS IBM version 20. Microbial load was expressed as log10 and reported as mean. The means were separated using Duncan multiple range test.