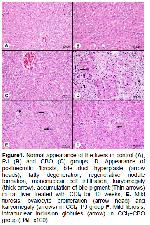
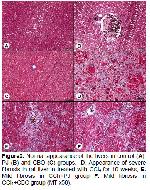
The present study demonstrates the protective and antioxidant effects of PJ and CBO aganist CCl4-induced chronic liver damage in rats. The reaction between CCl4 metabolites and polyunsaturated fatty acids causes formation of the covalent adducts with lipids and proteins that result in LPO and membrane damages with the consequent liver injury
24. The increase in liver MDA levels elicited by CCl4 has been reported to overproduct LPO, leading to hepatic tissue damage and failure of antioxidant defense mechanisms to prevent formation of excessive free radicals
25. In this study, elevated MDA levels observed in CCl4 administered rats indicate hepatic damage, and also decreased MDA levels after PJ and CBO treatment suggest the free radical scavening activity of these natural compounds. Previous studies have revealed that high antioxidant properties of pomegranate and its derivates, such as juice, flower, peel and seeds on liver damage induced by various hepatotoxic chemicals
26-30. It has been also reported that the antioxidant effect of flavanoids that found in PJ improve the process of regeneration and accelerating the repair mechanism of damaged cell membrane
30. Phytochemical screening of cinnamon bark has revealed the presence of flavonoids, glycosides, coumarins, alkaloids, anthraquinone, steroids, tannins and terpenoids
31. Phytoconstituents like the flavonoids
32, triterpenoids
33, saponins
34 and alkaloids
35 are known to possess hepatoprotective activity.
The main non-enzymatic antioxidant of cells is the GSH that involves in the metabolism of numerous toxic agents. GSH may block LPO by preventing free radical overproduction and by preserving cytochrome P450 36. In this study, CCl4 administration led to a significant decrease in the GSH level which can be an important factor in the CCl4 toxicity. However, long-term PJ and CBO administration to CCl4-treated rats significantly increased the hepatic GSH levels. It has been reported that the mechanism of hepatoprotection by PJ aganist CCl4 toxicity might be due to restoration of the GSH level 30 which is an agreement with our findings. These findings were also supported by the histopathological and TUNEL analyses of liver tissues in groups treated with PJ and CBO in the present study.
CAT and GSH-Px are the other cellular antioxidants and they catalyse H2O2 to water. In this study, CCl4 administration declined the activity of the antioxidant enzymes in liver, which is confirmed by previous study 37. PJ having different classes of polyphenols and flavonoids has been shown to prevent the reductions in GSH-Px and CAT acitivities by its positive effect on antioxidant enzyme activities in vivo 30,38. In addition, C. zeylanicum ethanolic extract 16 and C. vernum ethanolic or aqueous extract 13 consumption has been reported to improve significantly antioxidant enzyme activities in CCl4-treated rats. Treatment of rats with CCl4 significantly reduced the activities of liver GSH-Px and CAT at the rates of 81% and 70%, respectively in this study. However, treatment of the rats with the PJ and CBO prevented the CCl4-induced decreases in GSH-Px and CAT activities, which are comparable with the control values in the present study. This shows the protection provided by PJ and CBO consumption by rats depending on maintaining the levels of these enzymes even after CCl4 treatment.
It has been reported that oxidative stress is one of the pathologic mechanisms of hepatic fibrosis 39. The increased MDA levels result from the reaction between free radicals and polyunsaturated fatty acids under high oxidative stress 40 increase the collagen synthesis 39,41,42. The excessive levels of reactive oxygen species generated by CCl4-damaged liver cells have been reported to affect stellate cell activation and to enhance the production of the extracellular matrix, containing collagen 43. The antioxidant substances within the PJ have been alleged to decline the amount of connective tissue in liver by means of their positive effects on down-regulation of excessive expression of fibrogenic cytokines and collagenes 44. Flavonoids have counteractive effect on collagen accumulation that is the early stage of the fibrotic process 45, and they increase the activity of protective enzymes 46,47. It has been postulated that the beneficial strategies are the activation of antioxidase and the inhibition of LPO for preventing of early stage hepatic fibrogenesis 48. CCl4 has been reported to cause necrosis 28, fibrosis 30, inflammatory cell infiltration 49, fatty accumulation and degeneration of hepatocytes 16,49, increases in mitotic activity 50 and cirrhosis 51 in liver. Aforementioned histopathological lesions determined by different researchers are exactly similar to our findings found in the liver tissue after CCl4 treatment. Besides, in the present study, the CCl4-induced histopathological findings were significantly decreased by PJ and CBO treatment. The protective effect exerted by the PJ 28,30 and CBO 16 against CCl4-induced liver damage was confirmed by conventional histological examination. In the present study, CCl4 treatment caused ~8-fold increase in periportal fibrosis as compared to control. However, hepatic tissues of CCl4+PJ and CCl4+CBO groups showed consistent reduction in the liver fibrosis and the cirrhotic process. Especially, Masson's trichrome staining showed more regular liver architecture, in which only thin fibrous bands connecting portal areas were seen in CCl4+PJ and CCl4+CBO treatment groups compared to alone CCl4 group.
It has been reported that CCl4 causes apoptosis in liver 30,50,52. Apoptosis is the first step lesion observed in the hepatic tissue. Apoptosis and proinflammatory cytokine genes, which are inhibited during the engulfing of apoptotic bodies by macrophages 53, have been suggested to involve in liver fibrosis induced by CCl4 54,55. It has been reported that CCl4 substantially affect the cytokine-related genes 56. In addition, DNA fragmentation along with oxidative stress has been reported to play a crucial role in CCl4-induced hepatotoxicity leading increased apoptotic liver cells 57. There was an about 12-fold increase in apoptosis in CCl4 group in comparison to the control group in this study. However, we found that PJ and CBO consumption markedly decreased the number of TUNEL-positive cells in rats treated with CCl4. In the present study, increased LPO induced by decreased CYP activity after CCl4 administration might have possibly caused the liver histopathological damages and increase in the liver apoptotic index.
It was concluded that two herbal antioxidants, PJ and CBO, have ability to prevent the CCl4 damages in liver tissue rats. The hepatoprotective effects of PJ and CBO are possibly due to the reductions in liver fibrogenesis and apoptosis associated with the prevention of CCl4-induced oxidative stress. These actions of PJ and CBO are of significant clinical importance in the prevention of alcohol abusing- or other xenobiotics-induced liver damages show similarity to the CCl4-intoxication model.