Alternative treatment methods have been commonly used in treatment of diseases since ancient times. For many years the effects of herbal medication on wound healing have been reported. Herbal products seem to have effect with less or no toxicity comparing to the synthetic drugs
21-23. In this study, stimulatory effects of Pr and HP on burn wound healing were studied in clinical, biochemical, and histological terms.
Experimental animals such as rats have been used in wound healing studies 13,24,25. In this study, the burn wound model used is easy and reproducible.
In accordance with previous studies 2,14, the back skin of the rat seems to be an appropriate model for the study of burn wound healing and we used this model due to its simplicity.
Appropriate wound healing requires equilibrium between antioxidants and oxidative stress. Increase of reactive oxygen species inhibits wound healing, and also in a reduction in some free radical scavengers to cause tissue damage. Antioxidants increase the healing of wounds by decreasing the damage that is caused by free oxygen radicals 26-28.
Numerous antioxidants have been used in the treatment of wound both experimentally and clinically. Among them; Vitamins A, C, E, β-carotene, glutathione, SOD, dimethyl sulfoxide, melatonin, selenium, uric acid, ceruloplasmin, coumarine, ubiquinol, glycolic acid, and caffeic acid phenethyl ester could be mentioned 4,14.
Pr is a natural medication that has been used since ancient times. In the last decades, Pr has attracted investigators attention owing to several pharmacological and biological properties 3. It was notified that the antimicrobial 29 and anti-inflammatory activity 5 of Pr is owing to caffeic acid phenethyl ester. Some researchers 12,28, reported that antioxidative effect of Pr.
The time-dependent reductions in MDA level and statistical increments in all antioxidants on day 14 were determined in both control and Pr groups when compared with the values on day 7 (Table 1). This situation may be explained with the natural increase in wound healing by the time and subsequent improvements in oxidant/antioxidant balance. In addition, results of this study clearly demonstrated that Pr plays a role positively in healing of skin burn wounds by its anti-inflammatory and antioxidant effects.
It is reported that the HP extract has anti-inflammatory and antioxidant effects 6,7,11,30. In this study, MDA and SOD levels were significantly different in HP group compared to the control group on day 7 (P<0.001). A statistical increase was observed in only SOD activity between days 7 and 14 in HP group (P<0.05) (Table 1). However, it is believed that HP does not have strong antioxidant properties compared with Pr.
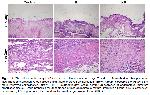
The accumulation of collagen demonstrates wound healing 31. Pr was used as an antiseptic and cicatrizant in burn wound treatment 4. It was reported that Pr extract have important anti-inflammatory properties and has reduced oedema in inflammation via this feature 5.
It was reported that HP has stimulating effects of fibroblast migration, collagen accumulation, and revascularisation 9,10,32.
In this study, on the 7th and 14th days, granulation tissue, collagen accumulation and epithelisation in animals in the Pr and HP groups were higher compared with the control group (P<0.05), as shown by the previous studies 9,32,33. In accordance with by the previous studies 3,4, it was found that the serious oedema observed in the control group decreased in the Pr and HP groups.
It is known that HP increased wound healing 33. Samadi et al. 34 showed that HP aided in healing caesarean wounds.
The results acquired in this study indicated that the antioxidant property of Pr was more than that of HP. Pr and HP were found to accelerate wound healing probably by increasing the formation of collagen deposition, epithelial and granulation tissue. It is also thought that antioxidant property of Pr contribute to the wound healing.
Consequently, results of this study remarked that Pr and HP skin creams could be applied in treatment of burn wounds and it may be an alternative to the traditional methods.