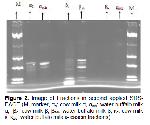
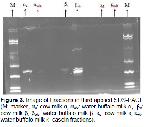
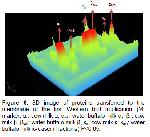
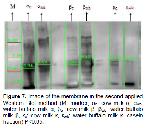
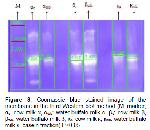
In this study, the general composition of cow milk and buffalo milk was determined and molecular weights of casein fractions was exhibited by SDS-PAGE and Western Blotting techniques.
The average fat values of cow and buffalo milk were found to be 3.63% and 8.60%, and the non fat dry matter values were 8.77% and 9.82%, respectively. These values were higher in buffalo milk. According to the results of the independent sample t-test statistical analysis; a significant difference was found between fat and non fat dry matter contents of cow and buffalo milk (P<0.05).
Salman et al. 14 showed that buffalo milk had a considerable amount of dry matter, fat, protein, lactose and ash content compared to cow milk, and that the calorific value in buffalo milk was significantly higher than that of cow's milk. Enb et al. 15 and Mahmood and Sumaira16 stated that the dry matter content of buffalo milk was more than that of cow's milk.
When the average protein values of cow and buffalo milk were compared, these values were 3.31% and 3.75%. According to the results of the independent sample t-test statistical analysis; a significant difference was found between the amount of protein of cow and buffalo milk (P<0.05). This result was similar reported by Salman et al. 14 and Mahmood and Sumaira 16.
In addition to calorific values, buffalo milk is a rich source of macro nutrients and has an important place in dairy products. Salman et al. 14 suggested that cow's milk with low fat compared to buffalo milk could be used as low-fat milk drinks or low-fat dairy products.
In general, milk has a complex structure with many chemical and physical components. Although milk of different animal species contains the same type of components, the amounts of these components are different. Within a given species, environmental factors such as genetic factors, climate and lactation period affect the composition 17.
In this study; according to SDS-PAGE and Western Blotting results of α-casein fraction, mean molecular weight values were found as 24.397 Da for cow's milk and 26.127 Da for buffalo milk. According to the statistical analysis findings, it was observed that there was a significant difference between the molecular weights of cows and buffalo milk α-casein fraction (P<0.05). Kuasar et al. 18 found that cow's milk αs1-casein and as2-casein molecular weights were 27 and 29 kDa, buffalo milk as2-casein molecular weight was 29 kDa.
In the study, mean values of β-casein fraction were 25.134 and 27.852 Da in cow and buffalo milk respectively. According to Independent Sample T-Test; it was determined that the difference between β-casein fraction molecular weights of cow and buffalo milk was significant (P<0.05). Kuasar et al. 18 found the molecular weights of β-casein fraction in cows and buffalo milk to be 24 and 25 kDa, respectively. The results obtained from the study were found to be relatively higher than the results of Kuasar et al. 18.
The average molecular weight of the κ -casein fraction was found to be 28.665 and 26.886 Da in cow and buffalo milk. The difference was statistically significant (P<0.05). In the study of Kuasar et al. 18, the molecular weight of the cow and buffalo milk κ-casein fraction was determined to be 22 kDa. It can be said that this result is higher compared to the study of Kuasar et al. 18 and this finding may be due to the difference in fractionation methods.
The molecular weight of buffalo milk casein fractions is higher than that of cow's milk and it affects the physicochemical and rheological properties of the milk products. Especially for the production of mozarella type cheese, cow and buffalo milk can be used as long as this information is shown on the label.
In the comperative study of Hussain et al. 5 about mozzarella cheese rheology made from cow and buffalo milk, when cow's milk was used (pH 6.7-6.5), the coagulation with rennet was slower than that of buffalo milk. It has been also concluded that the coagulum takes longer to form the maximum firmness and that the hydrolysis of the к-casein in cow's milk takes longer. Therefore the stability of the casein micelles is prolonged and the coagulum development is delayed. The casein micelles in buffalo milk rapidly increase the coagulum structure due to the fact that the fraction volume is higher than cow's milk. Therefore, a smoother structure is formed within the coagulum made from buffalo milk and it is stated that the coagulum obtained from buffalo milk is generally stronger and more frequent 5.
Analysis of milk protein expression, identification, characterization and quantification of milk proteins, determination of structure and modifications containing genetic variations, changes in phosphorylation levels (naturally occurring during milk processing and storage), glycosylation and other post-translational modifications (PTMs) will contribute to the development of proteomics in the determination of PTM domains on milk proteins, the acquisition of many valuable information for the dairy industry, and a better understanding of the structure and properties of buffaloes and other species' milk. In this respect, proteomic studies will guide the standardization and development of product quality 19.
In conclusion, the molecular weight of casein fractions in cow and water buffalo milk were significantly different. Casein micelles contribute to the physicochemical properties of milk and affect its functionality. It was concluded that the molecular weight of buffalo milk casein and its subunits is larger than that of cow's milk, it will improve the physicochemical properties of dairy products in a positive way and it will increase the yield. It was emphasised that the spread of buffalo milk will increase the quality and product diversity.
In addition, the importance of buffalo milk was emphasized with the study of buffalo milk casein and casein fraction molecular weight was found higher than cow's milk. The electrophoretic profiles of casein fractions in both types were revealed.