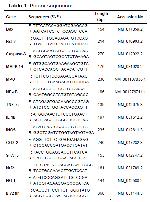
I/R, which is defined as the reduction of oxygen to tissues and subsequent restoration of blood flow, can lead to irreversible damage. Since skeletal muscle is the predominant tissue in the limb, it is more vulnerable to I/R injury. Although I/R generally occurs in acute situations and treatment with chemical agents is less preferred, in recent years, researchers have carried out numerous studies aimed at reducing I/R injury in skeletal muscle with various compounds
23. The current investigation aimed to assess the efficacy of morin, a compound with established antioxidant, anti-inflammatory, and anti-apoptotic properties, in mitigating skeletal muscle I/R damage. The obtained results demonstrate that morin significantly reduces I/R injury.
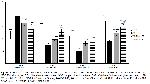
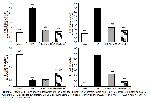
The balance of the oxidant state in the body is greatly influenced by antioxidant enzymes and substances24. Cells experience oxidative stress when there is a reduction in antioxidant activity and a depletion of antioxidant molecules, resulting in an elevation of oxidants25. I/R leads to a decrease in tissue antioxidant levels by causing excessive ROS formation. Ultimately, this decrease causes the antioxidant defense mechanism to be ineffective against oxidative tissue damage8. The susceptibility of membrane lipids in skeletal muscle to oxidation is significant. Ischemia and the accompanying reperfusion process give rise to the generation of reactive oxygen species, resulting in oxidative damage by upsetting the equilibrium between oxidants and antioxidants. The current study established that the concentration of MDA, a crucial marker of lipid peroxidation, increased following the initiation of I/R injury in skeletal muscle. Conversely, the administration of morin resulted in a reduction of MDA levels, with the extent of drop being contingent upon the dosage administered. Enzymatic antioxidants, including as SOD, CAT, and GPx, as well as the non-enzymatic antioxidant GSH, are integral components of the defense system against oxidative stress26. In a prior investigation, scholars unveiled that I/R injury instigated oxidative stress by means of free radical assault, resulting in substantial damage to skeletal muscle tissue. This detrimental effect was attributed to the diminished enzymatic activities of SOD, CAT, and GPx, as well as reduced amounts of reduced GSH27. The current investigation revealed that the administration of morin in rats with I/R damage resulted in a rise in GSH levels, with the extent of increase being dependent on the dosage supplied. The study revealed a reduction in the activities of SOD, CAT, and GPx enzymes in the group experiencing ischemia/reperfusion (I/R) damage. Conversely, the groups treated with morin exhibited an increase in the activities of these enzymes. The results of this research indicate that morin possesses the capacity to alleviate oxidative stress triggered by I/R injury in skeletal muscle. Furthermore, it highlights morin's potential to shield cells from harm by boosting the activities of antioxidant enzymes and increasing the levels of GSH.
A basic leucine zipper protein called Nrf2 may control the production of antioxidant proteins which protect against oxidative damage brought on by injury and inflammation. The substance is mostly distributed throughout the cytoplasm and forms a combination with Keap1. Keap1 serves as the inhibitory regulatory factor for Nrf2 and facilitates the degradation of Nrf2 via the process of ubiquitination. This mechanism enables the maintenance of Nrf2's physiological state at a low level of activity28. Concerning oxidative stress, Nrf2 migrates to the nucleus after undergoing phosphorylation and dissociation from Keap1. This translocation leads to the activation of downstream mechanisms that regulate the expression of genes such as HO-1, GPx, and NQO1. These genes, along with the associated antioxidant response element (ARE) sequence in their promoters, collectively contribute to the essential role of Nrf2 in safeguarding cells against inflammatory processes. HO-1 serves as the principal enzyme responsible for antioxidative and anti-inflammatory processes, which are initiated by the activation of Nrf229. In research conducted by Gendy et al.30, it was demonstrated that there was a decrease in Nrf2 and HO-1 mRNA expression levels in the context of I/R injury. Another research has shown that the injection of Fisetin, a kind of flavonoid, during I/R injury leads to an elevation in Nrf2 levels and an enhancement of HO-1 activity, thereby providing protection against oxidative damage. Hence, this research aimed to examine the potential antioxidant properties of morin in mitigating skeletal muscle I/R injury, focusing on its modulation of the Nrf2/HO-1 signaling pathway. The findings of the study indicate that the expression of Nrf2 and HO-1 was downregulated in skeletal muscle subjected to ischemia and subsequent reperfusion. However, the administration of morin resulted in an increase in Nrf2 expression, particularly in the I/R + Morin 100 group (P<0.001). This upregulation of Nrf2 led to an elevation in HO-1 levels, hence suggesting a reduction in oxidative stress.
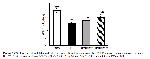
MPO belongs to the peroxidase superfamily and is mostly expressed in monocytes and neutrophils. Reactive species formed by MPO are principally involved in the process of phagocytosis, hence playing a crucial role in the antimicrobial action directed against a wide range of pathogens. Therefore, elevated MPO activity serves as a reliable marker for the presence of neutrophil infiltration in I/R damage31. Elevated levels of circulating MPO are associated with inflammation and oxidative stress32. The results of our study show that I/R injury upregulates MPO gene expression, but morin administration reduces MPO expressions by regulating neutrophil infiltration in skeletal muscle.
Besides oxidative stress, there is an increasing body of data suggesting that inflammation and cytokine activity play a crucial role in the development of skeletal muscle I/R damage33. NF-κB, a transcription factor of considerable importance, governs a multitude of biological processes such as cell cycle regulation, cellular migration, programmed cell death, and inflammatory responses. The investigation of the NF-κB pathway has significant therapeutic importance due to its ability to induce transcription of proinflammatory mediators, including COX-2, IL-1β, and TNF-α34. This phenomenon is one of the compelling evidence, which supports the link between oxidative stress and inflammation in the progression of ischemia/reperfusion.
In conjunction with proinflammatory cytokines, nitric oxide (NO) serves as a significant molecule involved in the mediation of tissue damage in the context of I/R injury. Inflammatory triggers, such as endotoxins, cytokines, and lipid mediators, result in an increase in iNOS expression35. Ince et al.36 have reported that skeletal muscle is impacted by NO produced by iNOS. Flavonoids play a crucial role in regulating modulation of inflammation pathways and cellular functions such as cell cycle signals. Similar to the studies in the literature, our results show that inflammatory markers are increased in I/R injury, and morin administration protects skeletal muscle by inhibiting cytokine activities. Based on these results, it can be said that the inflammation process in reperfused skeletal muscle after ischemia was suppressed by morin administration and the damage caused by inflammation was reduced.
Mitogen-activated protein kinases (MAPKs) have important activities as cellular signaling mediators37. The activation of MAPKs leads to an upregulation of gene expression, therefore inducing a diverse range of biological reactions, including the production of pro-inflammatory substances. MAPK 14, sometimes referred to as P38α, is well recognized as a pivotal modulator of the inflammatory response across several cellular lineages38. In their study, Qin et al.39 observed an elevation in the level of MAPK 14 after I/R damage. In a similar vein, our findings demonstrate that the expression of MAPK 14 is upregulated as a consequence of the heightened activation of NF-κB in the context of skeletal muscle ischemia-reperfusion damage. Conversely, the injection of morin has a suppressive effect on such activity while concurrently providing cellular protection against potential injury.
An integral part in several pathophysiological diseases, including inflammation, is played by STAT3, a transcription factor and member of the STAT family40. STAT3 translocation in the nucleus as a result of activation leads to increased NF-κB transcriptional activity, which contributes to the increased expression of cytokine genes41. Information on STAT3 gene expression in skeletal muscle I/R injury is limited in the literature. Pottecher et al.42 found out an increase in STAT3 activation in the case of skeletal muscle exposure to I/R. Similarly, the results obtained in the present study also indicate that in I/R injury, STAT3 gene expression increases depending on the transcriptional activity of NF-κB but decreases with the administration of morin depending on the dose.
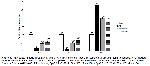
Apoptosis, commonly referred to as programmed cell death, is a natural process activated by various physiological and pathological factors. Bax and Bcl-2 are recognized as pivotal markers of apoptosis. Disturbance in the equilibrium between Bax and Bcl-2 results in an upsurge of cytochrome C levels within the cytoplasm, thereby initiating the activation of caspase-3 43. Caspase-3 is known as a good marker of apoptosis and initiates the apoptotic cascade by activating other caspase enzymes25. In a research conducted by Cheng et al.44, it was revealed that caspase-3 levels increased in skeletal muscle subjected to I/R injury. Additionally, a separate investigation reported that the limb I/R model resulted in elevated Bax levels and reduced Bcl-2 levels45. Our study's findings demonstrate that during I/R conditions, there is an upregulation in Bax and caspase-3 expressions, accompanied by a downregulation of Bcl-2 expression, ultimately leading to cellular apoptosis. Administration of morin at doses of 50 mg/kg b.w. and 100 mg/kg b.w. has shown significant efficacy in inhibiting cell death, preserving mitochondrial homeostasis, and maintaining membrane potential.
Excessive production of ROS leads to the irreversible oxidation of cellular macromolecules, including DNA, resulting in cellular damage. Autophagy is a crucial cellular degradation mechanism that facilitates the lysosomal breakdown of misfolded proteins and damaged or superfluous organelles 13. Beclin-1 is a skeletal protein of the PI3K complex and a good marker of autophagy. Based on the analyzes by Western blotting, I/R injury increases autophagy by damaging cells, as seen in Fig. 5. However, morin administration prevents this impact by reducing autophagy. Consistent with our results, researchers have found in the previous studie on the skeletal muscle that I/R injury increases autophagy46.
The pathophysiological process of ischemia/reperfusion injury is complex and significantly regulated by the oxidative stress response and the acute inflammatory response5. Inflammatory mediators such as ICAM-1 can aggravate and block micro vessels. Moreover, this phenomenon might result in the production of inflammatory mediators and cytokines via the activation of leukocytes. Additionally, the release of free radicals can lead to neuronal injury47. NF-κB can modulate gene expression levels of chemotactic factors, including ICAM, thus playing a critical role in cell inflammation and apoptosis. Calcium overload and oxidative stress can be induced by reperfusion following ischemia for further NF-κB activation. ICAM-1 plays a role in the inflammatory pathological process, as the expression level of ICAM-1 can be increased by various inflammatory factors48. Xiang et al.49 have shown an elevation in ICAM-1 levels in the context of skeletal muscle I/R damage. The findings obtained in our investigation also shown an elevation in ICAM-1 levels in the context of I/R damage, consistent with the existing literature. It was determined that the damage was reduced through the administration of morin, and ICAM-1 levels approached the control group levels.
In conclusion, the findings of the current research provide evidence that morin has protective properties against I/R damage in rat skeletal muscle. The protective effect of morin against I/R damage may be attributed to its anti-apoptotic, antioxidant, anti-autophagic, and anti-inflammatory qualities. However, before a therapeutic application is advised, further research must be carried out to validate these results.