After originating from v. jugularis externa at the entrance of the thoracic cavity in domestic mammals other than cats, vena cephalica runs caudoventrally in the sulcus pectoralis lateralis between m. pectoralis descendens and m. brachiocephalicus
5,7,9,10,17,22. In the present study, however, it was observed that v. cephalica was formed by the fusion of the middle 1/3 of v. jugularis externa and the two veins separating at the level of the thoracic cavity entrance at the level of the insertio of m. brachiocephalicus.
It is stated that v. mediana cubiti, originating from v. cephalica at the level of articulatio cubiti, terminates in v. brachialis superficialis in carnivores, in the caudal vein of v. brachialis pair in pigs, and in v. brachialis in ruminants in mammals3,7,12,14,42,46,47. Bozkurt22 states that v. cephalica joins with v. mediana through v. mediana cubiti in rabbits, while Özüdoğru16 states that they did not find the presence of this vein in rabbits. In this study, the presence of v. mediana cubiti was detected in rats in accordance with the literature data.
Some researchers stated that v. cephalica merged with v. mediana in the palmar of the antebrachium in rats. In this study, it was observed that v. cephalica run in the mediodistal direction after giving v. cephalica accessoria in the flexor of art. cubiti, formed 2-3 connecting branches with v. mediana, and the two veins showed a parallel run towards the distal. In rats, there are differences in the literature data since v. cephalica does not merge with another vein on the palmar surface of the antebrachium5,48.
It was stated that v. saphena medialis, which is one of the superficial veins of the pelvic limb, separated from the medial of v. femoralis in the distal direction and run together with a. saphena and n. saphenous in domestic mammals such as cattle, horses, cats and dogs4,7,47,49, rats5,50,51, and rabbits17,18,52. In the present study, the origin and run of v. saphena medialis were in parallel with the literature data5,17,18,50,53.
It was reported that v. saphena medialis can be found in pairs in pigs7,49, sheep and goats54 and rabbits18. In this study, it was determined that all the rats had a single v. saphena medialis. Some researchers18,50,53,55 reported that r. cranialis of v. saphena medialis gives many branches to the rete calcaneum in rats and rabbits. Greene5 and Popesko et al53 stated that in rats, the vein passed over the dorsal part of the foot and participated in the formation of v. digitalis dorsalis communis I. Some researchers5,17,48,50,53,56 also stated that r. caudalis of vena saphena medialis ended by dividing into v. plantaris lateralis and v. plantaris medialis at the distal end of the crus in rats and rabbits, and that it gave many branches to rete calcaneum during its run. In the present study, the run of r. cranialis and r. caudalis of v. saphena medialis and the branches it gave are in parallel with the literature data5,18,22,50,53.
Hebel and Stromberg57 and Greene5 stated that v. saphena lateralis originates from v. poplitea in rats. Popesko et al. 50 reported that the vein was formed by the union of v. ischiadica and v. caudalis femoris. In the present study, the data obtained on v. saphena lateralis in rats are in parallel with Popesko et al.s finding.
It was reported that vena saphena lateralis divided into r. cranialis and r. caudalis at the mid-crus level in rats, r. cranialis gave vv. digitales dorsales II, III, IV on the dorsal part of the foot, and ramus caudalis divided into r. lateralis and r. medialis again5. The data obtained in our study about v. saphena lateralis and its branches are in parallel with the literature.
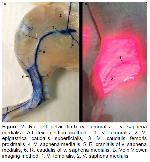
Vein viewer imaging method could not show that v. cephalica originated from v. jugularis externa in regio colli in rats. In the dissection, it was observed that the glandula mandibularis in this region was quite large and there was a dense fatty tissue around it. This is thought to be an obstacle to viewing with the vein viewer device. Imaging of vena cephalica with the vein viewer device showed parallelism with the latex findings down from regio brachii. While v. cephalica accessoria was detected in the distal 1/3 of the antebrachium with the device in rats, the entire run of the vein and the branches it gave to the dorsal foot could not be clearly viewed with the device. This is thought to be due to the fact that adipose tissue mass starting in the neck region progresses to the middle of the antebrachium.
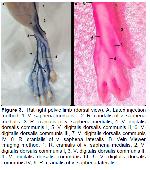
One of the superficial veins of the pelvic limb, v. saphena medialis and its branches were in parallel with the latex findings of the vein with the device. Vena saphena lateralis, which is often preferred as an injection site, was also viewed with vein viewer, and the thin branches leading to the fascia cruris were also viewed. The viewing of vv. digitales dorsales communes, which is the continuation of the vein, was clearer than the thoracic limb.
It is stated that the vein viewer device is preferred in humans, especially during peripheral intravenous catheterization, in order to make the veins visible58,59. In this study, it was determined that especially the pelvic limb superficial veins in rats were successfully viewed and could be used for catheter applications.
The findings obtained in this study show that the superficial veins especially of the pelvic limbs in rats are clearly viewed with the vein viewer device. In the thoracic limb, v. cephalica accesoria, which is one of the preferred veins for intravenous interventions, was viewed with the device. The viewed vein runs show parallelism with the data obtained by the latex injection method. In the light of the data obtained, the use of the vein viewer device as an alternative method in viewing of superficial veins in animals. Due to this fact, it would be advantageous to carry out various studies by applying the vein viewer device in veterinary applications with additional research being planned after this point.