Cd exposure remains a global public health problem
23. There is significant evidence that Cd exposure is associated with inflammation (neutrophil infiltration and Kupffer cell activation), non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, and necrotic hepatocellular death in humans
24,25. LIN found in natural essential oils shows many bioactive properties such as hepatoprotective, nephroprotective, anti-inflammatory, anticancer and antimicrobial. It has also been reported that LIN can be used as an adjuvant of antibiotics or anticancer drugs due to its protective effects
7. This study provides important evidence that LIN application against Cd-induced liver tissue damage has a hepatoprotective effect by suppressing inflammation and regulating SpX immunoreactivity.
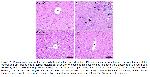
The increase in AST and ALT levels, which are frequently used clinically as liver function tests, indicates the deterioration of membrane integrity in hepatocytes26. Therefore, AST and ALT are also used as markers of liver tissue damage. It has been shown in many studies that Cd exposure significantly increases liver enzymes1,27,28. In this current study, consistent with previous studies, it was found that Cd augmented liver enzyme levels. However, many studies have reported that Cd causes widespread histopathological changes in liver tissue29,30. For example, it has been reported that Cd exposure causes hepatocyte degeneration, inflammatory cell infiltration, dilated sinosoids and necrotic cells in liver tissue1. Similarly, in this current study, it was shown that Cd application caused histopathological changes in liver tissues. On the other hand, it was determined that LIN application had a regulatory effect on Cd-induced increased liver enzyme levels and histopathological changes. These results are coherent with previous studies reporting that LIN application exhibits regulatory effects on liver enzyme levels and histopathological changes in many different hepatotoxic models such as carbon tetrachloride31, benzene 32, ischemia-reperfusion33 and high-fat diet34.
Cd accumulates in the liver and causes inflammatory reactions 28. Many studies have confirmed that inflammation is associated with Cd-induced toxicity35,36. In response to Cd toxicity, resident macrophages and Kupffer cells in the liver are activated. This triggers a complex network of inflammatory mediators. Thus, proinflammatory cytokines are (IL-1β, TNF-α, and IL-6) secreted in the liver37,38ğ. In a study conducted on rats, Cd caused a significant decrease in the level of IL-10 (anti-inflammatory cytokine) while increasing pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) in liver tissue39. Similarly, in this current study, IL-1β and TNF-α immunoreactivities were found to increase in Cd-induced liver tissue. The results obtained were consistent with previous studies reporting Cd-induced increases in pro-inflammatory cytokines in the liver1,28,40. One study reported that oleic acid has a liver-protective effect by reducing the levels of pro-inflammatory cytokines, which increase due to Cd40. LIN was reported to have a protective effect against peripheral inflammation caused by the endotoxin Salmonella typhimurium41. In addition, LIN suppressed nephropathy by exhibiting anti-inflammatory and antioxidant effects in a diabetic rat model42. However, LIN also inhibited TNF-α-induced inflammation in brain tissue endothelial cells43. It has also been reported that LIN inhibits proinflammatory pathways and cytokines induced by LPS44,45. Similarly, in this current study, LIN reduced the immunoreactivities of pro-inflammatory cytokines (TNF-α and IL-1β), which increased in Cd-induced liver tissue.
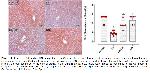
In homeostasis, adipokines (Adiponectin, Transforming growth factor beta, and IL10,) secreted by immune cells and adipocytes maintain physiological function, increase insulin sensitivity and inhibit inflammation in adipose tissues. The inflammatory environment that develops in fat tissues with obesity increases with the secretion of adipokines (IL6, resistin, leptin) released by adipocytes, infiltrated macrophages (M1) that differentiate into type 1 phenotype, and proinflammatory cytokines (IL6, TNFα and IL1β) by other inflammatory cells17,46,47. However, immune system cells (monocytes, neutrophils and macrophages) are known to express GALRs and play a role in inflammation48. It has been shown that SpX modulates inflammation in adipose tissues at the mRNA level when fed a fructose-rich diet and can improve the M1/M2 ratio by reducing the percentage of M1 macrophages49. In this study, SpX immunoreactivity decreased in Cd-induced liver tissue. Similarly, a recent study reported that endogenous SpX is susceptible to metabolic changes and SpX levels decrease in diseases such as obesity and diabetes. Additionally, the same study reported that SpX treatment showed many positive effects on metabolism, including reduction of lipid accumulation, fat mass, and inflammation50. Another study reported that SpX administration reduced IL-6 and TNF-α levels as well as lipid content in the liver of an experimental obesity and diabetes model51. Additionally, SpX application has been shown to regulate negative effects such as hepatocyte inflammation, necrosis, or bleeding in rats14. The current study results showed that SpX levels decreased in contrast to increased liver enzyme levels, histopathological changes, and proinflammatory cytokines in liver tissue due to Cd exposure. However, while LIN application had a regulating effect on liver enzyme levels, histopathological changes and pro-inflammatory cytokine levels, it also increased the Cd-induced decreased SpX immunoreactivity in liver tissue.
Casp-3 is an important cytoplasmic pro-enzyme that causes irreversible apoptosis when activated2. Studies have shown that Cd significantly increases Casp-3 levels in liver tissue1,27. However, a recent study reviewed the protective potential of LIN in a rat model of isoproterenol induced myocardial infarction. According to the results of this study, LIN showed a cardioprotective effect by increasing the level of Bcl2 (antiapoptotic), decreasing the levels of Nuclear Factor kappa B cytokines, IL-1β, TNF-α and IL-6, and apoptotic markers (Casp-3, Casp-9 and Bax)52. Similarly, in this study, it was detected that the increased Casp-3 immunoreactivity in Cd-induced liver tissues decreased with LIN application.
In this study, it was determined that Cd treatment increased liver enzyme levels, histopathological changes, and levels of inflammatory and apoptotic markers in liver tissues. It was also observed that Cd application reduced SpX immunoreactivity in liver tissue. On the other hand, it was determined that all Cd-induced negativities were regulated by LIN. In Cd-induced hepatotoxicity, LIN application showed a hepatoprotective effect by regulating proinflammatory cytokines and SpX levels. These results indicate that LIN, a monoterpene, may be a potential therapeutic candidate against heavy metal toxicity, but further and comprehensive studies are needed to determine its detailed mechanisms.