In recent years, it was shown that BPA can adversely affect the endocrine system, even at low doses
11. Previous studies have shown that BPA can induce excessive insulin stimulation in the endocrine pancreas and contributing to insulin resistance
12,33. It has been reported that BPA may increase the risk of developing type II diabetes by directly affecting pancreatic cells, impairing insulin and glucagon secretion, inhibiting cell growth, triggering cell apoptosis, affecting muscle, liver, and fat cell function, and activating insulin resistance
14,33,34. Similarly, it was reported that 100 mg/kg intraperitoneal injection of BPA into female mice decreased the size and cell number of islets of Langerhans
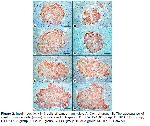
35. In the present study, statistical differences were determined between the groups in terms of islet number, diameter, and area. Accordingly, we determined that the number of islets decreased by 50.29%, 17.34%, 37.68% and 20.81% in BPA, BPA+CoQ10, BPA+FUL, and BPA+ALA groups, respectively, compared to the control group. In the BPA, BPA+CoQ10, BPA+FUL, and BPA+ALA groups, islet diameters decreased by 28.42%, 20.08%, 14.51%, and 6.09% and islet areas decreased by 40.79%, 28.61%, 25.41% and 5.27%, respectively. These observed changes in islets of Langerhans were evaluated as an indication that BPA can directly affect pancreatic cells and lead to the development of type I diabetes. It was reported that insulitis developed at the end of 11 weeks and type 1 diabetes developed at the end of 28 weeks in offspring born from pregnant mice administered BPA at a dose of 10 mg/l in daily drinking water
14.
A previous study determined that no histopathological changes occurred in the islets of Langerhans of male rats exposed to 5, 50, or 500 μg/kg/day oral doses of BPA for 8 weeks. However, in the same study, the percentage of insulin-positive β cells in the islets of Langerhans increased significantly in the group administered BPA at a dose of 500 μg/kg/day compared to the other groups2. Another study in which rats were administered Bisphenol F (BPF) at 20, 100, or 500 mg/kg/day for 28 days, insulin positive β cell index values decreased in BPF groups compared to the control group33. In the present study, no histologic changes were observed in the pancreas of male rats after oral administration of 25 mg/kg/day BPA for 7 weeks, and insulin positive β cell index was significantly decreased in BPA-treated groups compared to control and antioxidant-only groups. It was determined that β cell index decreased by 9.72%, 6.47%, 8.77%, and 6.52% in BPA, BPA+CoQ10, BPA+FUL, and BPA+ALA groups, respectively, compared to the control group. BPA dose, animal species, and exposure time seem to be the main factors determining whether histopathologic changes will occur in the pancreas.
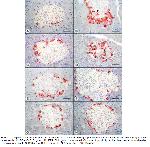
It has been reported that BPA causes oxidative stress and has detrimental effects on organs such as the pancreas, liver, brain, kidney, and testis35. In addition, it was reported that BPA injection at a dose of 2mg/kg/day in male rats caused a decrease in antioxidative effective enzymes in the pancreas at the end of four weeks and an increase malondialdehyde levels, a product indicative of oxidative stress36. In the present study, caspase 3 positivity increased by 265.37%, 141.30%, 321.89%, and 223.16% in the BPA, BPA+CoQ10, BPA+FUL, and BPA+ALA groups, respectively, compared to the control group. In the islets of Langerhans in rats, β-cells make up 60-80% of the total number of cells, α-cells 15-20%, δ-cells less than 10%, and PP-cells approximately 1% (37-39). It was reported that a significant increase in caspase 3 positivity was observed in the islets of Langerhans in 11-week-old offspring of female mice exposed to BPA (3000 μg/kg/day) during pregnancy14. In the present study, we interpreted that the decreases in the number, diameter, and area of islets detected in the BPA-exposed groups compared to the control group may be due to oxidative stress-based apoptotic cell deaths in islets, especially β cells.
CoQ10 is a fat-soluble compound that acts as a coenzyme in important enzymatic reactions during energy production in cells and can be found in every cell40,41. Although CoQ10 is at a lower concentration than other antioxidants in plasma, it is the first antioxidant to react with plasma oxidants. It is also known to play a role in the regeneration of other antioxidants40,42. When CoQ10 was administered with metformin in diabetic rats, it was found to reduce the number of apoptotic cells in the pancreas and provided a significant improvement in insulin staining intensity in islets43. In addition, it was reported that CoQ10 reduced changes such as interstitial edema, inflammatory cell infiltration, and acinar necrosis in an experimental pancreatitis model in rats44. In rats with diabetes induced by sirolimus, CoQ10 administration at a dose of 20 mg/kg/day for two weeks caused a decrease in the number of TUNEL positive cells in islets of Langerhans43. In another study, there was no significant difference observed in terms of β cell area determined by histopathological and immunohistochemical insulin positivity between the group administered 100 mg/kg/day dose of BPA alone and the group administered 10 mg/kg/day dose of CoQ10 with the same dose of BPA for 14 days45. In the present study, CoQ10 administration at a dose of 40 mg/kg/day significantly ameliorated the apoptotic effects of BPA on the endocrine pancreas.
FUL treatment was found to be effective for the prevention of oxidative stress against increased lipid peroxidation and changes in antioxidant enzyme activities in doxorubicin-treated male rats46. In male rats with acute cholangitis, development of acute pancreatitis characterized by necrosis, degeneration, edema, and thrombosis in the acinus was observed, and degenerative changes in the pancreas were suppressed by oral or intraperitoneal 0.5 mg/kg FUL administration for 3 days47. In the present study, it was surprisingly noted that FUL administration at a dose of 8 μg/kg/day together with 20 mg/kg/day BPA exacerbated the apoptotic effect of BPA in islets. In addition, it was found to have no effect on morphometric changes. Consistent with the present study, the cell number and volume in the islets decreased by approximately 50% at the end of 5 days in the endocrine pancreas of rats with streptozotocin (STZ)-induced diabetes; 25.00 mg/kg FUL treatment daily with STZ injection did not improve the condition in the pancreas and caused further deterioration of periductal inflammatory changes48.
It has been emphasized that ALA has suitable properties to treat tissue damage caused by free oxygen radicals due to its strong antioxidative effects49. It was reported that administration of 100 mg/kg/day ALA for 8 weeks to STZ-induced diabetic rats prevented necrotic and degenerative destruction in endocrine pancreatic cells50. Consistent with previous studies, we found in the present study that administration of 70 mg/kg/day ALA together with 25 mg/kg/day BPA for 7 weeks significantly improved BPA-induced morphometric changes in the pancreas and decreased the number of apoptotic cells in islets. In previous studies, ALA was shown to prevent oxidative damage and cell death by scavenging oxygen radicals, metal ion chelates, and various mechanisms protective against lipid peroxidation50-52.
In conclusion, while long-term BPA administration in rats did not result in observable histopathological changes in the endocrine pancreas, significant morphometric alterations, decreased insulin secretion, and apoptosis in islet cells were identified in the present study. Notably, CoQ10 and ALA exhibited partial protective effects against the impact of BPA on the endocrine pancreas. Given the absence of studies exploring the effects of ALA and FUL on BPA-induced pancreatic tissue damage, it is suggested that future research should focus on unraveling the detailed interaction of these antioxidant substances with BPA.