In this study, it was aimed to investigate NLR, LMR, and serum Fe levels and the relationship between these data in calves with different diarrhea etiologies. Many inflammatory biomarkers have been investigated in calf diarrhea, but there are barely any studies in which the levels of NLR and LMR were investigated in calves with diarrhea.
It has been reported that depending on the severity of the infection, clinical symptoms such as reluctance to move, anorexia, increased body temperature, increased respiratory rate and skin fold test time, and decreased sucking reflex may develop in calves with diarrhea19. In this study, higher body temperature, respiration and pulsation rates were obtained in calves in the diarrheal groups than in the control group calves. It is thought that the reason for the increase in body temperature in calves with diarrhea may be due to the effects of infectious pathogens and the severity of dehydration20. It is thought that the reason for high respiration in calves with diarrhea may be due to a compensatory polypnea to return the pH to normal levels, and tachycardia may occur due to faster heart working to ensure more intense on pumping of blood to the periphery in case of hypovolemia caused by dehydration21.
The main factors of infectious causes of calf diarrhea can be listed as viruses (rotavirus, bovine viral diarrhea virus, and coronavirus), bacteria (Escherichia coli and Salmonella spp.), and protozoa (like Eimeria zuernii), as well as non-infectious factors such as stress, sudden ration changes, and imbalances in ambient humidity22,23.
Early confirmation of inflammatory conditions is of utmost importance for cattle herd health, welfare, and early diagnosis of diseases. Nowadays, WBC, differential WBC counts, and acute phase protein levels are mostly used for the detection of inflammatory conditions in cattle24. WBC counts are higher in calves less than 6 months old as compared to adult cattle. However, this level returns to normal levels after 3 years of age 25. Neutrophilia occurs primarily during the recovery period of intense inflammatory conditions or during mild to moderate inflammatory conditions. Neutrophilia has also been reported to occur in infectious diseases, neoplastic conditions, non-inflammatory conditions, or tissue damage. Neutropenia occurs in cattle during acute, intense inflammatory conditions such as sepsis, mastitis, metritis, salmonella infections, pneumonia, and peritonitis5. In a study in which Fe levels were investigated for the confirmation of inflammatory status in cattle, it was reported that band NEU levels increased significantly in mastitis and RPT disease compared to cattle in the control group14. In a study in which a bovine respiratory disease model was established for viral and bacterial infections, it was reported that WBC and NEU counts were significantly increased after experimental intratracheal exposure of Mannheimia haemolytica (M. haemolytica) agent compared to the group that did not receive the M. haemolytica exposure26. The reason for this situation was attributed to the fact that intratracheal M. haemolytica can rapidly initiate acute reactions within 24 hours after exposure, as stated by Corrigan et al.27. In study conducted on calf diarrhea, it was reported that higher WBC and NEU values were obtained in the diarrhea groups compared to the control group 28.
In this study, it was observed that the WBC and NEU values in the diarrhea group were significantly higher than those in the control group (P<0.001). This is believed to be due to the rapid onset of acute inflammatory reactions, as stated by Corrigan et al.27. In addition, the strong correlation (rho=0.815; P<0.01) and regression findings (R=0.88; R2=0.78) between WBC and NEU support this finding.
In human medicine, it is stated that NLR is a useful biomarker in determining sepsis29. In the field of veterinary medicine, studies on the NLR ratio are mostly investigated in tumoral diseases and different disease states in dogs10,30,31. It has been understood in the literature review that studies on NLR are less common in cattle. It has been reported that an increase in the level of WBC, NEU, LYM and NLR in calves infected with M. haemolytica infection is caused by an increase in the infection load that triggers an inflammatory response32. It has been reported that the higher NLR level in cattle with acute toxic mastitis compared to the control group may be due to the increased number of neutrophils with the effect of increased acute inflammatory response33.
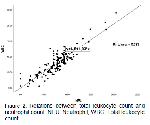
In this study, similar to the above studies, higher WBC, NEU, and NLR levels were found in the diarrhea groups compared to the control group. This may be due to the increase in the number of NEUs as a result of the increase in the acute inflammatory response caused by infectious agents as mentioned by Braun et al. 33. The highest NLR value was found in group-2 (E. coli group). In addition, the strong correlation between WBC and NEU (rho=0.815; P<0.01) and the strong degree regression data between WBC and NEU (R=0.88; R2=0.78) proved that acute inflammation was more severe in group-2 compared to the other groups.
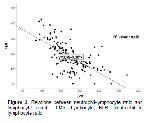
Lymphocytosis is not commonly seen in cattle, but it has been reported to occur under conditions such as chronic viral and pyogenic infections. It has been stated that lymphopenia occurs in corticosteroid administration and stress or diseases and pathological conditions such as acute viral and bacterial infections, and rarely immunodeficiency34. Circulating leukocytes show an increase in the number of neutrophils in the face of stressful situations, while the number of lymphocytes decreases35. LMR is an important marker of systemic inflammatory response 36. To the best of the authors knowledge, there have been very few studies investigating LMR in cattle. It has been reported that NLR was decreased and LMR was increased with the administration of hydrolyzed tannin extract in a study on calf health and this may be attributed to the anti-inflammatory and immunomodulatory properties of tannin extract37. In inflammatory bowel disease, low level of LMR has been reported to be positively correlated with advanced disease38. Similarly, low level of LMR has been reported to be associated with poor prognosis in lymphomas in dogs and cats39,40. It has been reported that the presence of moderately pronounced lymphopenia haematologically is directly proportional to the severity of the disease and the damage caused by this severity 41. It has been reported that monocytes have an active role in the control of infection as well as in the pathogenesis of inflammatory diseases42. In a study on breast cancer in human medicine, it was reported that elevated Fe-Monocyte-Lymphocyte ratio and low LMR ratio associated with low Fe in individuals with Coronavirus-19 (COVID-19) disease indicate poor prognosis43,44. Different studies on giardiosis have reported that increased activation of macrophages plays an active role in the digestion of giardia trophozoites45,46.
In this study, it was determined that group-5 (Giardia spp.) had the highest value in terms of MON numbers (P<0.05). It was observed that the group with the lowest LMR values was formed in group-5, and this value created a significant difference (P<0.05). This may be due to the highly active role of macrophages (hence monocytes) against Giardia spp. infections as stated by Hill and Pohl45. In addition, the statistically insignificant correlation between the level of LMR, which is an inflammatory marker, and Fe also supports this finding (rho=0.135; P>0.05).
Since acute phase proteins such as haptoglobin or serum amyloid A require special equipment for use in clinical practice, it is extremely important to be able to determine the inflammation status more quickly in cattle health and to use alternative biomarkers with less cost, since it is time consuming and impractical. Fibrinogen is an acute phase protein that can be easily measured manually in blood14. The levels of Fe, which is one of the trace elements, decrease rapidly in inflammatory diseases in cattle. Moreover, Fe can be easily measured together with other parameters with standard biochemical devices47. Fe stimulates immune system activation by affecting the function of immune system cells. This effect is manifested by activation of NEU, LYM and macrophages in the presence of sufficient levels of Fe. In inflammatory conditions, the storage of Fe in reticuloendothelial cells increases with the effect of acute phase proteins such as hepcidin, alpha-1 antitrypsin and cytokines and blood Fe levels decrease accordingly. This is a protective mechanism that inhibits the proliferation of pathogenic microorganisms that utilize Fe48. Serum Fe concentration has been used to confirm inflammatory status in horses13. However, in terms of cattle health, it is seen that relatively limited data are obtained for the confirmation of inflammatory conditions of Fe. It has been reported that serum level of Fe is significantly decreased in various inflammatory diseases47, cattle with traumatic peritonitis and mastitis14,49, bovine respiratory disease (BRD) and Fe can be used as an inflammatory marker50. Tsukano et al.50 also reported that the number of experiments was not sufficient for Fe to be used as an inflammatory marker and the etiology and severity of BRD were not determined. In contrary to these studies, it was stated that there was no significant change in serum levels of Fe within 24 hours in cattle with endotoxemia model and its use as a prognostic marker was not significant. The reason for this situation has been shown as not knowing the beginning of the infection period in animals with endotoxemia and the small number of animals used in the research51.
In this study, although similar results were obtained to the studies mentioned above, different results were also observed. First of all, the reason for this difference may be the differences in experimental design between the above-mentioned studies and this study, the inability to create a possible sample in terms of disease severity in the experimental group animals of the above-mentioned studies, and the differences in the immune status of the animals depending on their age. In addition, one of the most important difference is that the animals in the experimental group in this study were a suitable sample in accordance with the diarrhea protocol and the number of animals used in the study was sufficient. The formation of significant data between the diarrhea and control groups in terms of serum Fe levels in this study supports the importance of the number of subjects used in the study as stated by Tsukano et al.50.
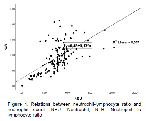
Boyuk et al.52 reported that the NLR rate was higher and Fe levels were lower in the patient groups in Helicobacter pylori infection and a negative correlation was observed between NLR and Fe. This negative correlation has been suggested to occur since Fe increases the proliferation of immune system cells53. In severe COVID-19 infection, low level of Fe, which negatively correlates with high NLR with excessive increase in inflammatory response, has been reported to be indicators of increased rate of mortality54. In the present study, the levels of Fe were found to be significantly lower in all diarrhea groups compared to the control group (P<0.01). The lowest Fe level was observed in group-2 (E. coli group). In addition, when the correlation findings were examined, a negative correlation (rho=-0.390; P<0.01) was obtained between the NLR and Fe levels. Therefore, it was concluded that the inflammatory response was significantly higher in group-2, which had the highest NLR level and the lowest Fe level, compared to the other diarrhea groups. This may be explained by the enhancing effect of Fe on the proliferation of immune system cells53 or by the severity of the inflammation (high NLR level) in the E. coli group54.
The present study has several limitations. First, although the groups were selected according to broadly different aetiologies, it is evident that more sensitive analyses are needed to determine the etiology with certainty. However, Rotavirus, Coronavirus, E. coli (K99), and Cryptosporidium spp. are reported as the causative agents of calf diarrhea in the first 30 days. The prevalence of these agents was reported to be 6.69% for Cryptosporidium spp., 2.84% for bovine coronavirus, and 1.64% for Enterotoxigenic E. coli55. Therefore, in the current study, a rapid diagnosis kit was used to determine the most common factors in neonatal calf diarrhea. Secondly, blood and stool samples were taken from the calves only once. It is seen that the prognostic evaluation of the study with repeated measurements can yield meaningful results. Finally, it is predicted that valuable results can be obtained with studies investigating hematological inflammatory markers (NLR, LMR), Fe, inflammatory cytokines, and acute phase biomarkers together.
In this study, it was observed that NLR, LMR, and Fe levels may be found at different levels according to the etiological agents in calves with diarrhea and may explain the inflammatory state. It was determined that NLR levels in calves with E. coli diarrhea were higher than in other calves with diarrhea, but LYM, MON, and Fe levels were lower. Therefore, it was concluded that the inflammatory response was more intense in calves with E. coli diarrhea according to NLR and Fe levels used as inflammatory markers. However, in order to verify these data, it is extremely important to investigate the reality of this situation with repeated measurements and experimental studies by forming patient groups on larger scales.