Case History: An 11-month-old Akkaraman sheep, one of 240 animal in a flock, underwent necropsy at the Department of Veterinary Pathology, Fırat University.The sheep were free-ranging and fed on pasture grass, barley, and hay. The animal had two weeks of history of weakness, loss of appetite, weight loss and submandibular swelling.
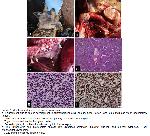
Gross Findings: Gross examination showed the tumoral masses in the thymus extending anteriorly to the submandibular region and posteriorly to the mediastinal lymph nodes. The tumor was grayish in color with an irregular yellowish appearance, and it was circumscribed and non-encapsulated (Figure 1a). Typical swelling and congestion were also present in the submandibular region of the neck. The cross-sectional areas of the tumors were gray with irregular yellow color (Figure 1b). The tumoral mass extend into posterior mediastineum and spread into lymph nodes (Figure 1c).
Büyütmek İçin Tıklayın |
Figure 1: Morphological features of thymic lymphoma.
a. Enlargement due to tumor development in submandibular area, primary tumor (arrow) and submandibular lymph nodes(arrow heads).
b. The cut surfaces of tumoral lymph nodes and primary tumor in cervical region.
c. Thymic tumor and mediastinal lymph nodes.
d. Dense lymphocytic proliferations divided by thin fibrous septa.
e. The lymphocytes had intermediate nuclear size, dispersed chromatin, indistinct nucleoli, and minimal cytoplasm; T-cell lymphoblastic lymphoma.
f. CD20 immunoreactivity in tumor cells. |
Histopathological and Immunohistochemical Studies: Tissue samples collected during necropsy were fixed in a solution of formalin, processed routinely, and stained with hematoxylin and eosin (H-E) for light microscopic examination. Immunohistochemistry (IHC) was also performed using an autostainer (Dako Autostainer Universal Staining System; Dako, Carpinteria, CA) following standard procedures. BDTM Retrieval A solution was used for high-temperature antigen retrieval, and methanol containing 3.0% H2O2 was used to block endogenous peroxidase activity. Mouse monoclonal against human CD3 and rabbit polyclonal against human CD20 (Dako M7051 and A0452) were used as primary antihuman antibodies and applied for 30 minutes at room temperature.
Microscopic examination of the tissue samples revealed a diffuse population of neoplastic cells with a polymorphic morphology and of lymphoid origin. These cells formed sheets, and were mainly composed of lymphocytes. Thin, irregular collagenous septums were present within the tumoral mass (Figure 1d). The cells had an irregular nucleus, anisokaryosis, fine chromatin, a marked nucleolus, and a scant cytoplasm (Figure 1e). The cells had a high nucleus/cytoplasm ratio, with frequent mitotic cells and apoptotic bodies. Lymphoblastic cells were also observe.
The immunohistochemical staining results indicated that the tumor cells were positive for CD20 (Figure 1f), and negative for CD3.
Molecular Studies: Nested PCR procedure for detecting enzootic bovine leukosis virus (EBLV) was performed as previously described (6).
DNA Extraction and PCR: The template DNA was extracted from mediastinal lymph nodes using the DNA/RNA purification kit (Zymo-research, CA, USA) according to the manufacturers instructions First and the second PCR steps were performed using 2X PCR master mix (Thermo Fisher, MA, USA) with env5032/5608r external primers and env5099/5521r internal primers, respectively. The amplification procedure for both PCR steps was performed as follows: 40 cycles, denaturation at 94 °C for 30 sec, annealing at 62 °C for 30 sec, and elongation at 72 °C for 1 min to amplify 598 and 444 bp products. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping primers were used as an extraction control7. A blank reaction consisting of primers but no DNA template was included to serve as a reagent control.
PCR results, with env5032/5608r and env5099/5521r, showed occurrence of no amplicons. Similarly, no amplicon was detected in the negative control. Approximately 700 bp product was obtained with the GAPDH primers. Therefore, the tissue samples were negative for EBLV.



