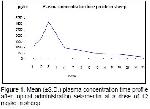
Pharmacokinetic studies on selamectin
1,14,15
usually carried out in cats and dogs. In sheep, goats and
cattle, there was no study conducted. In this study, there
were evaluated mean plasma concentration levels, C
max,
AUC, MRT and T
max values during following 35 days
after topically administration of selamectin with a single dose 12 mg/kg in sheep and goats. Animal species, sex,
route of administration, and feeding, body-fat ratio,
physico-chemical structure and formulation of drug were
known on pharmacokinetic of selamectin
1,8,14-22.
As a result of the analysis, for sheep mean plasma
concentration, Cmax, AUC, MRT and Tmax values
determined as 1427.27±90.52 pg/mL, 4.78±0.61ng/mL,
810.35±115.95 ng.h/mL, 10.86±0.89 days and 72 hours,
respectively and for goats plasma concentration, Cmax,
AUC, MRT and Tmax values determined as
1195.03±70.81 pg/mL, 3.27±0.52 ng/mL, 664.42±72.62
ng.h/ml, 10.46±0.74 days and 72 hours, respectively.
In a study conducted by Sarasola et al.15, after
topically administration of selamectin Cmax and Tmax
values were determined as 86.5±34.0 ng/mL and 3 days
in dogs, respectively and for cats 5513±2173 ng/mL and
15 hours, respectively.
In a study conducted by Dupuy et al.1, after
topically administration of selamectin with 6 mg/kg dose
Cmax, AUC, MRT, Tmax values determined as 12.72±5.13
ng/mL, 192.08±63.85 ng.d/mL, 12.55 days, 4.86±3.56
days at male dogs, respectively and 22.65±11.95 ng/mL,
370.97±146.87 ng.d/mL, 12.55 days, 5.2±1.87 days for
female dogs, respectively.
As a result, selamectin was topically administired for
treatment and control of parasitic diseases of sheep and
goats, mean plasma concentration, Cmax and AUC values
were found higher in sheeps compared to goats and no
significant difference were identified between goats and
sheep for MRT and Tmax values.
This study has contributed to pharmacokinetic values
of selamectin at sheep and goats. We believe that new
studies must be conducted for dose and dose limits,
planned a variety of pharmacokinetic studies, revealing
drug interactions and determination of residual period of
selamectin at sheep, goat and cattle.