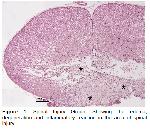
Experimental models have been developed in recent years to unravel the pathophysiology and effects of spinal cord injuries, the cellular and molecular mechanisms that underlie tissue regeneration and repair in the injured spinal cord, and to monitor the injury process
25. Most injuries do not completely damage the spinal cord. Primary damage has been described by four main characteristic mechanisms. These are (a) impact and permanent compression, (b) impact and temporary compression, (c) irritation, and (d) laceration/ transection. Compression and compression types are the most common types in spinal cord injuries
25,26. In order to evaluate treatment efficacy and associated mechanisms in spinal cord injuries, a weight-drop contusion method was developed. After performing laminectomy at level T8, Ko et al.
27 and Lv et al.
28 induced experimental spinal cord injury by dropping a 10g weight from a height of 5 cm onto the spinal cord surface. The spinal cord injury generation method used in the present study is similar to the method used in the studies of Ko et al.
27 and Lv et al.
28 (Figure
1).
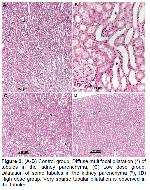
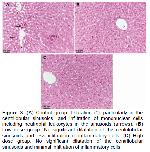
Spinal cord injury is a devastating event that results in significant physical disabilities for affected individuals. Aside from local damage to the spinal cord, patients with spinal cord injuries suffer from various complications including multiple organ dysfunction and failure as a result of systemic inflammatory responses. Injured patients are likely to develop neurogenic pain, depression, lung damage, cardiovascular disease, liver damage, renal dysfunction, urinary tract infection, and increased susceptibility to pathogen infections, all of which impede functional recovery and may even pose a life-threatening situation29-31. In the current study, lesions occurred in the kidneys, and liver of rats (Figure 2A-B, 3A). These conditions were histopathologically demonstrated. The lesions in the liver and kidneys observed in the study shows similar characteristics as described by Gris et al.29, Bao et al.30, and Sun et al.31.
Spinal cord injury not only affects motor and sensory functions but also leads to serious and long-term complications due to impaired nerve conduction to vital organs. Autonomic innervation of the kidney is interrupted due to spinal cord injury. Consequently, renal arterial blood flow, sodium and potassium excretion, and renal plasma flow are decreased, leading to renal dysfunction32-34. In their study, Kandhare et al.35 found that spinal cord injury-induced rats displayed a change in glomerular permeability due to kidney damage. In the studies by Shunmugavel et al.36 and Akakin et al.37, degeneration of renal tubules was observed in spinal cord injury-induced rats. Besides, Sakarcan et al.38 observed enlargement in Bowman's cavity, atrophy in glomeruli, and degeneration in tubules in spinal cord injury-induced rats. The findings of the above-mentioned studies are align with the histopathology results (Figure 2) of our study in the kidney tissue.
Liver function is regulated by autonomic parasympathetic innervation from the brain stem and sympathetic innervation from the thoracic spinal cord. Hence, spinal cord injury at or above the thoracic levels disrupts the main regulatory mechanisms for hepatic functions. Furthermore, the liver even has a major role in initiating and prolonging systemic inflammation after spinal cord injury39,40. In the study by Hundt et al.41, leukocyte infiltration was formed in the liver after the experimental spinal cord injury in rats, and in the study by Mohamed et al.40, dilatation and cellular infiltration in the sinusoids due to liver injury in spinal cord injury-induced rats were determined. Moreover, in the study by Goodus et al.42, leukocyte cell infiltrations were also observed in the liver in spinal cord injury-induced rats. In the present study, the histopathological changes in the liver (Figure 2) are similar to those observed by Mohamed et al.40, Hundt et al.41, and Goodus et al.42.
The pathophysiology of spinal cord injury comprises a series of destructive events that occur acutely or chronically, including ischemia, oxidative stress, inflammatory changes, apoptosis, necrosis, and locomotor dysfunction. A variety of therapeutic strategies are being developed to overcome neurodegenerative events and reduce secondary neuronal damage. Despite continued research into neuroprotective and neurodegenerative therapies, no treatment has been demonstrated to provide full recovery in spinal cord injuries. Thus, researchers are still searching for new medical therapies for the treatment of spinal cord injuries43-45. For a successful outcome in spinal cord injuries, drugs such as vasopressors, steroids, anti-inflammatory agents, and rehabilitation therapies should be combined with a consideration of the complex pathophysiology of the disease46. Shilajit is a compound used in conventional medicine that exhibits anti-inflammatory, analgesic, antioxidant, and neuroprotective activities47,48. Likewise, in this study in which different doses of shilajit were used, its positive effects on the liver and kidney were also proven histopathologically and biochemically. Despite the minimal pathological changes observed in the liver and kidneys in the groups administered high-dose shilajit (250 mg/kg), the pathological changes were moderate when administered at a low dose (150 mg/kg), whereas the pathological changes were advanced in the untreated group (Figure 2-3). The results of serum biochemistry in the present study (Table 1) provide evidence of spinal cord injury-induced damage to the kidney and liver tissue in rats and histopathological findings showing the healing effect of shilajit on these tissues. Compared with the control group, the decrease in ALT and AST serum concentrations, and increase in TP (P<0.05) and ALB (P<0.001) levels in the groups treated with low (P<0.05) and high (P<0.001) dose shilajit reflect functional liver recovery and the healing role of shilajit. AST and ALT in particular are important liver enzymes that are found in higher concentrations in the cytoplasm. Elevated AST and ALT are directly proportional to acute injury40,49. Additionally, BUN and creatine enzymes35,39, which are among the parameters indicating renal function, were lower (P<0.05) in the shilajit-treated groups compared to the control group, which biochemically proves the histopathological changes occurring in the kidneys due to spinal cord injury in shilajit-treated rats. Furthermore, CK and LDH enzymes, which are markers of skeletal muscle injuries50, decreased more significantly (P<0.05) in the serum levels of the high shilajit-treated group in the present study, which biochemically reveals the tissue damage inhibitory property of this substance.
Direk et al.10 demonstrated that atrophy of kidney glomeruli and degeneration of tubules occurred in spinal cord injury-induced rats. Cyclotrichium origanifolium (mountain mint) and Thymbra spicata L. var. spicata (zahter) they used in their studies partially prevented kidney damage. This damage caused degeneration in the kidneys, dilation in the Bowman space, and atrophy in the glomerulus in spinal cord injury-induced rats, as reported by Sakarcan et al.38 in their study. It was shown that riboflavin had a protective effect against these damages in the kidneys in the studies conducted. Similarly, in the present study, low-dose shilajit treatment offered a partial protective effect on kidney damage in spinal cord injury-induced rats compared with the control group, whereas this was more pronounced in high-dose shilajit treatment, and an effect with normal kidney histology was observed. This suggests that shilajit administration at a dose of 250 mg/kg may prevent kidney damage after spinal cord injury better than a 150 mg/kg dose.
Mohamed et al.40 showed that liver damage occurred in spinal cord injury-induced rats and revealed that liver damage decreased with granulocyte colony-stimulating factor (G-CSF) used for treatment. In their study, Bao et al.51 used anti-CD11d to reduce the damage in the treatment of liver damage after experimental spinal cord injury. In the current study, it has been confirmed through both histopathology results and changes in liver enzyme levels that the use of a high dose of Shilajit (250 mg/kg) significantly reduces the liver damage resulting from spinal cord injury when compared to the control group. Low-dose shilajit administration (150 mg/kg) partially prevented liver damage following spinal cord injury. This suggests that shilajit at a dose of 250 mg/kg to be used after spinal cord injury may prevent liver damage better than the used protocol at a dose of 150 mg/kg.
In conclusion, the protective effect of the shilajit compound against liver and kidney dysfunctions due to spinal cord injury in rats was investigated. In this study, it has been revealed histopathologically and biochemically that administration of the Shilajit significantly suppressed the formation of the lesions in liver and kidney with its anti-inflammatory properties after spinal cord injury in rats. In order to gain a better understanding of the mechanism of action of shilajit, further experimental studies need to be conducted.